
Interpretation:
The structure of the compound with molecular formula –
Concept Introduction:
The structure of 2,4-dibromo-3-methoxytoluene is as follows –
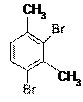
The compound has two methyl groups and two Bromine groups. Methyl groups are activating groups (electron releasing groups) and enhance the electron density in benzene and so the electrophilic substitution reaction on the benzene moiety occurs with ease. On contrary, Bromine groups are deactivating groups. Presence of high electronegative atom like Bromine decreases the electron density in benzene due to its electron withdrawing effect.
Activating groups are known as ortho- and para- directing groups because these groups when present on benzene ring increases the electron density of benzene at ortho and para positions. So they direct the incoming electrophile towards ortho and para position during electrophilic substitution reaction.
Deactivating groups are known as meta-directing groups because these groups when present on benzene ring decreases the electron density of benzene at ortho and para positions and so the incoming electrophile is directed towards meta position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
ORG.CHEM EBOOK W/BBWILEY PLUS>CUSTOM<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





