
Interpretation:
The
Concept introduction:
Chromatography is the technique used to identify the components of a mixture. The division of the distance covered by the component of a compound to the distance covered by solvent in the chromatography technique is known as the
Answer to Problem 1ASA
The
Explanation of Solution
The chromatograph for a mixture that is prepared by a student is shown below.

Figure 1
The modified chromatogram with proper divided parts is shown below.
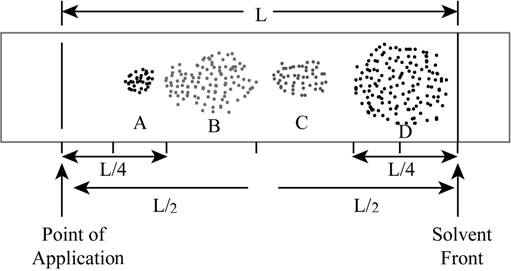
Figure 2
According to the above shown chromatogram, four compounds A, B, C and D are present in the mixture.
The total distance travelled by the solvent is
The total distance travelled by the compound A is
The total distance travelled by the compound B is
The total distance travelled by the compound C is
The total distance travelled by the compound D is
The expression to represent the
To calculate the
To calculate the
To calculate the
To calculate the
Therefore, the
The
Want to see more full solutions like this?
Chapter 2 Solutions
CHM 111/112 LAB MANUAL >C<
- 1. What is the retention time of molecule 9?2. What happens to the peak area if the sample amount is doubled?3. If run time is stopped at 5.5 mins, will molecules 8 & 6 be separated and quantified?4. If the flow rate was doubled, will 8&6 be separated and quantified?5. Does a single peak at time T in the chromatogram indicate only one compound is eluted?arrow_forwardHow might the Rf values of colorless substances be determined? That is, how could the colorless spots be located? (for paper chromatography)arrow_forwardThe following Rf values were computed from a normal phase chromatography experiment. Arrange them from most polar to least polar. I - 0.25 II - 0.84 III - 0.56 IV- 0.67 Group of answer choices II > IV > III > I I > III > IV > II I > II > III > IV IV > II > III > Iarrow_forward
- In column chromatography, dyes with higher polarities tend to attach to which of the forbwing? A. THF-Acetone B. Silica C. Acetone D. Tetrahydrofuranarrow_forwardPreparing a Thin Layer Chromatography experiment there are some common mistakes. Explain what happens in this situation: Sample spots too bigarrow_forwardIn an experiment of Reverse Phase chromatography using C-18 stationary phase and a mixture of methanol/ethyl acetate/acetic acid, a researcher tried to separate the 3 compounds shown below. Which compound would have the lowest Rf and which the highest Rf in this experiment? Explain your answer and show any relevant structures.arrow_forward
- I found the Rf value in chromatogram A but I’m unsure how to find the lowest point for Barrow_forwardThin layer chromatography experiment. Show calculations for the Rf of each spot you observed.arrow_forwardHow does ammonia, dimethylglyoxime (DMG), and 8-hydroxyquinoline (8HQ) interact with your cations, leading to the evolution of colored species? Context: Paper chromatography experiment (separation of inorganic cations) with a 9:1 acetone/ HCl solventarrow_forward
 Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning



