
Mastering Chemistry with Pearson eText - Standalone Access Card - for Basic Chemistry (5th Edition)
5th Edition
ISBN: 9780134270210
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 2.101UTC
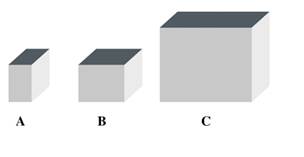
Consider the following solids. The solids A, B, and C represent aluminum, gold, and silver. If each has a mass of 10.0 g, what is the density of each solid? (2.7)
Density of aluminum = 2.70 g/mL
Density of gold = 19.3 g/mL
Density of silver = 10.5 g/mL
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following pairs have a relationship that is most similar to composition?
Group of answer choices
1.A toy car and Car.
2.A car and an engine.
3.A Honda Civic and Car.
4.A lock and a key.
Mr. Naresh works in a multinational company. He is stressed due to his hectic schedule. Mr. Amit, his friend, comes to know that he has started taking sleeping pills without consulting the doctor. Mr. Amit requests ‘ Naresh to stop this practice and takes him to a Yoga centre. With regular Yoga sessions, Mr. Naresh is now a happy and relaxed man. ‘
After reading the above passage, answer the following questions:
(i) Write the values shown by Mr. Amit.
(ii) Which class of drugs is used in sleeping pills?
(iii) Why is it not advisable to take sleeping pills without consultation with the doctor?
If 125,000 capsules can be made from 0.425 kg of ibuprofen, how many capsules can be made from 850g?
What amount in microliters of peppermint oil would be contained in each capsule, if 50 mL of peppermint oil is used to manufacture 50,000 capsules?
A patient receives 500 nanograms of alfacalcidol each day for 30 days. How much alfacalcidol does the patient receive in the 30 days, expresses as milligrams?
Chapter 2 Solutions
Mastering Chemistry with Pearson eText - Standalone Access Card - for Basic Chemistry (5th Edition)
Ch. 2.1 - Give the abbreviation for each of the following:...Ch. 2.1 - Give the abbreviation for each of the following:...Ch. 2.1 - Prob. 2.3QAPCh. 2.1 - State the type of measurement in each of the...Ch. 2.1 - State the name of the unit and the type of...Ch. 2.1 - Prob. 2.6QAPCh. 2.1 - Prob. 2.7QAPCh. 2.1 - Prob. 2.8QAPCh. 2.2 - What is the estimated digit in each of the...Ch. 2.2 - What is the estimated digit in each of the...
Ch. 2.2 - Identify the numbers in each of the following...Ch. 2.2 - Identify the numbers in each of the following...Ch. 2.2 - Identify the measured number(s), ifany, in each of...Ch. 2.2 - Identify the exact number(s), if any, in each of...Ch. 2.2 - Prob. 2.15QAPCh. 2.2 - Prob. 2.16QAPCh. 2.2 - Prob. 2.17QAPCh. 2.2 - Prob. 2.18QAPCh. 2.2 - Prob. 2.19QAPCh. 2.2 - Prob. 2.20QAPCh. 2.2 - Prob. 2.21QAPCh. 2.2 - 2.22 Write each of the following in scientific...Ch. 2.2 - Prob. 2.23QAPCh. 2.2 - Prob. 2.24QAPCh. 2.3 - Prob. 2.25QAPCh. 2.3 - Prob. 2.26QAPCh. 2.3 - Round off each of the following measurements to...Ch. 2.3 - Round off each of the following measurements to...Ch. 2.3 - Prob. 2.29QAPCh. 2.3 - Prob. 2.30QAPCh. 2.3 - Prob. 2.31QAPCh. 2.3 - Perform each of the following calculations, and...Ch. 2.3 - Prob. 2.33QAPCh. 2.3 - Perform each of the following calculations, and...Ch. 2.4 - Prob. 2.35QAPCh. 2.4 - In a French car, the odometer reads 22269. What...Ch. 2.4 - Prob. 2.37QAPCh. 2.4 - Prob. 2.38QAPCh. 2.4 - Prob. 2.39QAPCh. 2.4 - Prob. 2.40QAPCh. 2.4 - Prob. 2.41QAPCh. 2.4 - Prob. 2.42QAPCh. 2.4 - Prob. 2.43QAPCh. 2.4 - Prob. 2.44QAPCh. 2.4 - Prob. 2.45QAPCh. 2.4 - Prob. 2.46QAPCh. 2.4 - For each of the following pairs, which is the...Ch. 2.4 - Prob. 2.48QAPCh. 2.5 - Why can two conversion factors be written for an...Ch. 2.5 - Prob. 2.50QAPCh. 2.5 - Prob. 2.51QAPCh. 2.5 - Write the equality and two conversion factors for...Ch. 2.5 - Prob. 2.53QAPCh. 2.5 - Prob. 2.54QAPCh. 2.5 - Prob. 2.55QAPCh. 2.5 - Write the equality and two conversion factors, and...Ch. 2.5 - Write the equality and conversion factors, and...Ch. 2.5 - Prob. 2.58QAPCh. 2.5 - Prob. 2.59QAPCh. 2.5 - Prob. 2.60QAPCh. 2.6 - When you convert one unit to another, how do you...Ch. 2.6 - Prob. 2.62QAPCh. 2.6 - Prob. 2.63QAPCh. 2.6 - 2.64 Perform each of the following conversions...Ch. 2.6 - Prob. 2.65QAPCh. 2.6 - Prob. 2.66QAPCh. 2.6 - Prob. 2.67QAPCh. 2.6 - Use metric conversion factors to solve each of the...Ch. 2.6 - Prob. 2.69QAPCh. 2.6 - Prob. 2.70QAPCh. 2.6 - Prob. 2.71QAPCh. 2.6 - Using conversion factors, solve each of me...Ch. 2.6 - Prob. 2.73QAPCh. 2.6 - Using conversion factors, solve each of the...Ch. 2.6 - Prob. 2.75QAPCh. 2.6 - Prob. 2.76QAPCh. 2.7 - Prob. 2.77QAPCh. 2.7 - Determine the density (g/mL) for each of the...Ch. 2.7 - Prob. 2.79QAPCh. 2.7 - Prob. 2.80QAPCh. 2.7 - Prob. 2.81QAPCh. 2.7 - Prob. 2.82QAPCh. 2.7 - Prob. 2.83QAPCh. 2.7 - Prob. 2.84QAPCh. 2.7 - Prob. 2.85QAPCh. 2.7 - Prob. 2.86QAPCh. 2.7 - Prob. 2.87QAPCh. 2.7 - Solve each of the following problems: A glucose...Ch. 2 - Prob. 2.89FUCh. 2 - Prob. 2.90FUCh. 2 - Prob. 2.91UTCCh. 2 - Prob. 2.92UTCCh. 2 - Prob. 2.93UTCCh. 2 - Prob. 2.94UTCCh. 2 - Prob. 2.95UTCCh. 2 - Prob. 2.96UTCCh. 2 - Prob. 2.97UTCCh. 2 - Prob. 2.98UTCCh. 2 - Prob. 2.99UTCCh. 2 - Prob. 2.100UTCCh. 2 - Consider the following solids. The solids A, B,...Ch. 2 - Prob. 2.102UTCCh. 2 - Prob. 2.103UTCCh. 2 - Prob. 2.104UTCCh. 2 - Prob. 2.105AQAPCh. 2 - Prob. 2.106AQAPCh. 2 - A dessert contains 137 25 g of vanilla ice cream....Ch. 2 - Prob. 2.108AQAPCh. 2 - Prob. 2.109AQAPCh. 2 - Prob. 2.110AQAPCh. 2 - Prob. 2.111AQAPCh. 2 - Prob. 2.112AQAPCh. 2 - Prob. 2.113AQAPCh. 2 - Prob. 2.114AQAPCh. 2 - Prob. 2.115AQAPCh. 2 - A graduated cylinder contains 155 mL of water. A...Ch. 2 - Prob. 2.117AQAPCh. 2 - Prob. 2.118AQAPCh. 2 - Prob. 2.119AQAPCh. 2 - Prob. 2.120AQAPCh. 2 - Prob. 2.121AQAPCh. 2 - Prob. 2.122AQAPCh. 2 - Prob. 2.123AQAPCh. 2 - Prob. 2.124AQAPCh. 2 - Prob. 2.125CQCh. 2 - Prob. 2.126CQCh. 2 - Prob. 2.127CQCh. 2 - Prob. 2.128CQCh. 2 - Prob. 2.129CQCh. 2 - Prob. 2.130CQCh. 2 - Prob. 2.131CQCh. 2 - Prob. 2.132CQCh. 2 - Prob. 2.133CQCh. 2 - Prob. 2.134CQCh. 2 - Prob. 2.135CQCh. 2 - Prob. 2.136CQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A heated lead ball is added to 158 grams of water in a coffee cup calorimeter. If the ball loses 47.35 °C of heat and the water gains 1.3 °C of heat, what is the mass of the lead ball? (3 sf)arrow_forwardThe following nutrition information is listed on a box of crackers: Serving size 0.50 oz (6 crackers) Fat 4 g per serving Sodium 140 mg per serving a. If the box has a net weight (contents only) of 8.0 oz, about how many crackers are in the box? b. If you ate 10 crackers, how many ounces of fat did you consume? c. How many servings of crackers in part a would it take to obtain the Daily Value (DV) for sodium, which is 2400 mg?arrow_forwardThe energy needed to keep a 75-watt lightbulb burning for 1.0h is 270kJ. Calculate the energy required to keep the lightbulb for 5.0h in each of the following energy units. joules Express your answer using two significant figures E= ? J kilocalories Express your answer using two significant figures E= ? kcalarrow_forward
- The energy needed to keep a 75-watt lightbulb burning for 1.0h is 270kJ. Calculate the energy required to keep the lightbulb burning for 5.0h in each of the following energy units. Kilocalories Express your answer using two significant figures E=? Kcalarrow_forwardCalculate the percent difference between x2 and x4 according to the equation below. (Assume x2 to be the accepted value.) Record the calculation in your notes. x2 = 100.4 kJ x4 = -100.4 kJ %difference = experimental value - accepted value x 100% accepted valuearrow_forward13. A sample of 15.00 g of a hydrated sodium sulfate salt contains 7.05 g of water. The value of the "x" in the hydrated salt is: NaSO4 . xH2O Answers: a)7 b)10 c)1 d)3 e)5arrow_forward
- 6.) What is the ideal body weight of an individual which is 5 feet tall? a. 170kg b. 162 kg c. 140kg d. 152 kg 7.) As a student your physical activity is considered which of the following? a. bed rest b. light c. moderate d. sedentary 8.)This element regulates several important physiologic and biochemical processes including neuromuscular excitability, blood coagulation, secretory process, membrane integrity, and plasma membrane transport a. calcium b. magnesium c. iron d. potassium 9.) A micro mineral which is an essential component of blood a. Cobalt b. chromium c. molybdenum d. iron 10.) Fiber requirements are provided by which of the following? a. Meat b. Milk c. sweets d. fruits and vegetablesarrow_forwardAt the end of the experiment, it was discovered that the thermometer had not been calibrated. When it was calibrated, it was found that the thermometer read 0.50 C low. What effect would thus thermometer reading have on the reported change in H neoutzn calculated above?arrow_forwardA prescription reads 2 capsules per day taken after meals. Each capsule of the drug product contains 12.5 mg of drug. The number of capsules to be dispensed to the patient who requires a 50,000 mcg dose is _____.arrow_forward
- Which of the following values for R2 shows the strongest correlation between the X and Y values in a data set? 0.0184 0.0047 0.500 0.981 0.9678arrow_forwardA food contains 15 grams protien, 20 grams carbs, 8 grams of fat per seving. You consumed 2.5 servings of this food, how many Kcals were consumed?arrow_forwardA glass of skim milk supplies 0.1 mg of iron, 8.5 g of protein, and 1 g of carbohydrates. A quarter pound of lean red meat provides 3.4 mg of iron, 22 g of protein, and 20 g of carbohydrates. Two slices of whole-grain bread supply 2.2 mg of iron, 10 g of protein, and 12 g of carbohydrates. If a person on a special diet must have 19.9 mg of iron, 136 g of protein, and 116 g of carbohydrates, how many glasses of skim milk, how many quarter-pound servings of meat, and how many two-slice servings of whole-grain bread will supply this? skim milk glasses meat quarter-pound servings whole-grain bread two-slice servingsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY