
Interpretation:For the given system, whether the heat or work flow across the boundary between system and surroundings or not needs to be determined.
Concept Introduction:
An adiabatic procedure is one which takes place so vastly that no heat transfer takes placebetween the system, or within the system and surroundings.If the heat is added at constant volume the heat energy goes completely into increasing the internal kinetic energy of the substances.
Answer to Problem 2.1CP
On the resistor work is done and this work is done within the surrounding. The liquid’s temperature is changed by 1 0C. Hence due to the variation in temperature between system and surroundings, the flow of heat takes place across the boundary between surrounding and system.
Explanation of Solution
Suppose there is an adiabatic system containing a heating water and coil against a constant atmospheric pressure P atm.
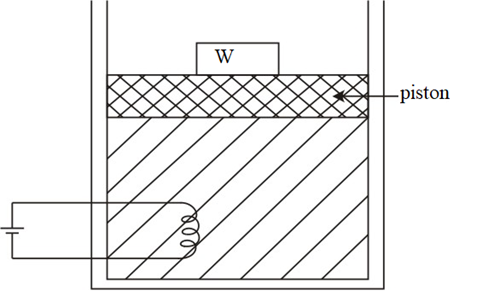
The temperature increases when electrical energy is introduced within the system. As per the law of
Here,
Adiabatic container is the system that means that no heat is exchanged from the system as well as its surroundings.
Hence,
On the resistor work is done and it is done within the surroundings.
The liquid’s temperature is changed by 1 0C. Hence due to the variation in temperature between system and surroundings, the flow of heat takes place across the boundary between surrounding and system.
Want to see more full solutions like this?
Chapter 2 Solutions
Physical Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





