
Concept explainers
(a)
Interpretation:
Lewis structure of
Concept Introduction:
Lewis structures represent covalent bonds and describe valence electrons configuration of atoms. The covalent bonds are depicted by lines and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons are estimated for each atom.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around the
symbol of the element to satisfy the octet (or duplet) for each atom. - Add charge on the overall structure in case of polyatomic cation or anion.
(a)
Explanation of Solution
Hence, 6 pairs are to be allocated to form the Lewis structure of
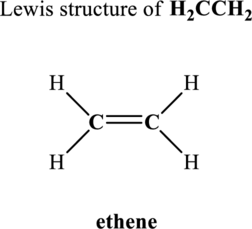
Similarly, total valence electrons is sum of the valence electrons for each atom along with a charge in
Hence, 8 pairs are to be allocated to form the Lewis structure of
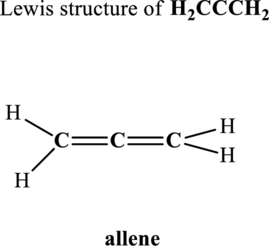
Likewise, total valence electrons in
Hence, 10 pairs are to be allocated to form the Lewis structure of
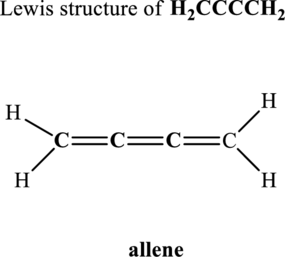
(b)
Interpretation:
The hybridization at each carbon in
Concept Introduction:
The table that relates the steric number with hybridization is as follows:
(b)
Explanation of Solution
In the structure of
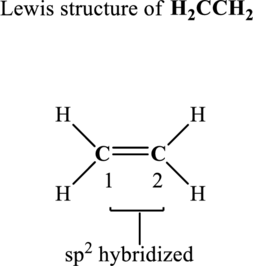
The terminal carbon atoms at positions 1 and 2 are trigonal planar with 3 as steric number hence the hybridization of these carbon atoms is
In the structure of
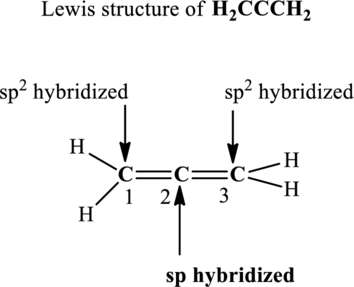
Since the steric number around central carbon is 2 therefore, the hybridization of carbon atom located at positions 2 is
In the structure of
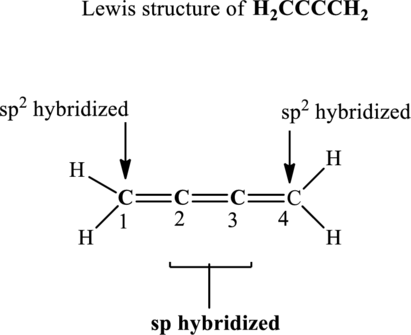
Central carbon atoms have effectively two bond pairs as each double bond is regarded as one single bond pair. Since the steric number around central carbon atoms is 2 therefore, the hybridization of carbon atoms located at positions 2 and 3 is
The terminal carbon atoms at positions 1 and 4 are trigonal planar with 3 as steric number hence the hybridization of this carbon is
(c)
Interpretation:
The type of bonds that connect carbon atoms in
Concept Introduction:
A single bond is always associated with sigma bonds. Molecules that have all atoms connected by sigma bond exclusively are stated to be in
A double bond and carbocation are associated with
A triple bond or central carbon structure similar to allene is always associated with
(c)
Explanation of Solution
In
In
In
(d)
Interpretation:
The bond angles in
Concept Introduction:
Refer to part (c).
(d)
Explanation of Solution
A single bond is always associated with sigma bonds. Molecules that have all atoms connected by sigma bond exclusively are stated to be in
A double bond and carbocation are associated with
A triple bond or central carbon structure similar to allene is always associated with
Thus the bond angles can be illustrated as follows:
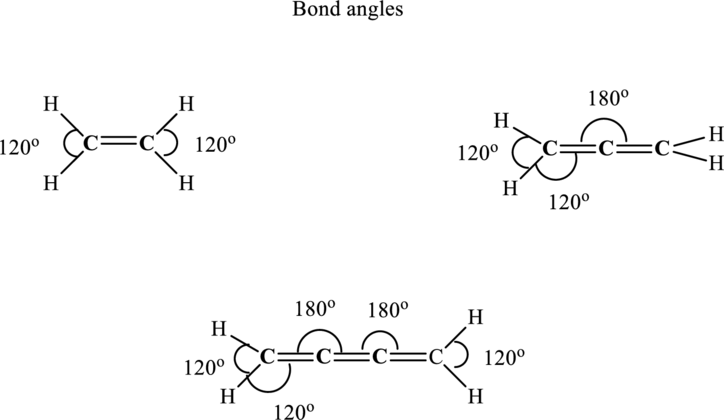
(e)
Interpretation:
Whether all hydrogen atoms lie in the same plane or not in
Concept Introduction:
Refer to part (c).
(e)
Explanation of Solution
In allene, the central carbon that is double bonded to each carbon in different planes is
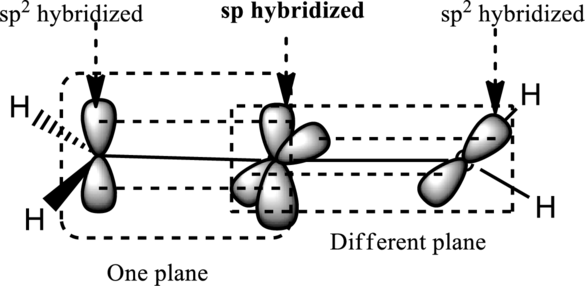
Thus the terminal hydrogen atom on carbon at position 1 is in a different plane than carbon at position 3 in case of
(f)
Interpretation:
Orientation of hydrogen atoms at end of chain has to be determined.
Concept Introduction:
In allene, the central carbon that is double bonded to each carbon in different planes is
(f)
Explanation of Solution
For structures with even number of carbon atoms, for example, consider the structure:
Here, the relative position of the hydrogen atom is indicated as follows:
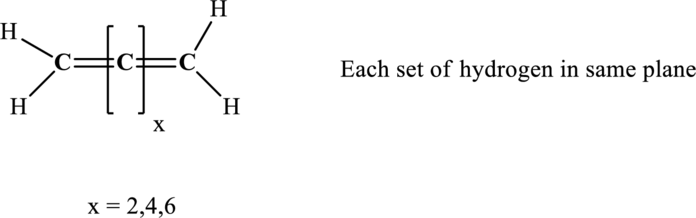
For structures with an odd number of carbon atoms, for example, consider the structure:
Here, the relative position of the hydrogen atom is indicated as follows:

Thus structures with even a number of carbon atoms as in
Want to see more full solutions like this?
Chapter 2 Solutions
CHEMICAL PRIN. (LL)+ 2 SEM SAPLING >BI<
- Formamide, HC(O)NH2, is prepared at high pressures from carbon monoxide and ammonia, and serves as an industrial solvent (the parentheses around the O indicate that it is bonded only to the carbon atom and that the carbon atom is also bonded to the H and the N atoms). Two resonance forms (one with formal charges) can be written for formamide. Write both resonance structures, and predict the bond angles about the carbon and nitrogen atoms for each resonance form. Are they the same? Describe how the experimental determination of the HNH bond angle could be used to indicate which resonance form is more important.arrow_forwardIt is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardGamma hydroxybutyric acid, GHB, infamous as a date rape drug, is used illicitly because of its effects on the nervous system. The condensed molecular formula for GHB is HO(CH2)3COOH. (a) Write the Lewis structure for GHB. (b) Identify the hybridization of the carbon atom in the CH2 groups and of the terminal carbon. (c) Is hydrogen bonding possible in GHB? If so, write Lewis structures to illustrate the hydrogen bonding. (d) Which carbon atoms are involved in sigma bonds? In pi bonds? (e) Which oxygen atom is involved in sigma bonds? In pi bonds?arrow_forward
- Aspirin, or acetylsalicylic acid, has the formula C9H8O4 and the skeleton structure (a) Complete the Lewis structure and give the number of bonds and bonds in aspirin. (b) What is the hybridization about the CO2H carbon atom (colored blue)? (c) What is the hybridization about the carbon atom in the benzene-like ring that is bonded to an oxygen atom (colored red)? Also, what is the hybridization of the oxygen atom bonded to this carbon atom?arrow_forwardA 0.325 g sample of a gaseous hydrocarbon (compound containing carbon and hydrogen only) occupies a volume of 193 mL at 749 mmHg and 26.1°C. Determine the molecular mass, and write a plausible Lewis structure for this hydrocarbon.arrow_forwardWhat is the hybridization of the two central atoms in ethane?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




