
The Science and Engineering of Materials (MindTap Course List)
7th Edition
ISBN: 9781305076761
Author: Donald R. Askeland, Wendelin J. Wright
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 2.6P
(a)
To determine
The number of atoms of aluminum contained in one square inch.
(b)
To determine
The number of atoms per 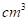 in lead and lithium.
in lead and lithium.
Concept Introduction
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An atom crystallizes in FCC crystal lattice and has a density of 10 g/ cm 3 with unit cell edge length of 100 pm. Calculate the number of atoms present in 1 g of the crystal.
If the atomic radius of lead, which has an FCC crystal structure, is 0.175 nm, calculate the volume of its unit cell in cubic meters.
1. Show that the atomic packing factor for HCP is 0.74.2. Calculate the radius of Palladium atom given that PD has an FCC crystal structure, a density of 12.0 g/cm3, and an atomic weight of 106.4 g/mol.
Chapter 2 Solutions
The Science and Engineering of Materials (MindTap Course List)
Ch. 2 - Prob. 2.1PCh. 2 - Prob. 2.2PCh. 2 - Prob. 2.3PCh. 2 - Why is it important to consider the structure of a...Ch. 2 - Prob. 2.5PCh. 2 - Prob. 2.6PCh. 2 - Prob. 2.7PCh. 2 - Prob. 2.8PCh. 2 - Prob. 2.9PCh. 2 - Prob. 2.10P
Ch. 2 - Write the electron configuration for the element...Ch. 2 - Prob. 2.12PCh. 2 - Prob. 2.13PCh. 2 - Prob. 2.14PCh. 2 - Prob. 2.15PCh. 2 - Prob. 2.16PCh. 2 - Prob. 2.17PCh. 2 - Prob. 2.18PCh. 2 - Prob. 2.19PCh. 2 - Prob. 2.20PCh. 2 - Prob. 2.21PCh. 2 - Prob. 2.22PCh. 2 - Prob. 2.23PCh. 2 - Prob. 2.24PCh. 2 - Prob. 2.25PCh. 2 - Prob. 2.26PCh. 2 - What are the bonding mechanisms in thermoplastics?Ch. 2 - Prob. 2.28PCh. 2 - Materials such as silicon carbide (SiC) and...Ch. 2 - Prob. 2.30PCh. 2 - Prob. 2.31PCh. 2 - Prob. 2.32PCh. 2 - Prob. 2.33PCh. 2 - Prob. 2.34PCh. 2 - In order to increase the operating temperature of...Ch. 2 - Prob. 2.36PCh. 2 - Prob. 2.37PCh. 2 - Prob. 2.38PCh. 2 - Prob. 2.39PCh. 2 - Prob. 2.40PCh. 2 - Would you expect MgO or magnesium to have the...Ch. 2 - Would you expect (Al2O3) or aluminum to have the...Ch. 2 - Prob. 2.43PCh. 2 - Prob. 2.44PCh. 2 - Steel is coated with a thin layer of ceramic to...Ch. 2 - Prob. 2.46PCh. 2 - Prob. 2.47PCh. 2 - Prob. 2.48PCh. 2 - Prob. 2.49PCh. 2 - Prob. 2.50PCh. 2 - Prob. 2.51DPCh. 2 - Turbine blades used in jet engines can be made...Ch. 2 - You want to use a material that can be used for...Ch. 2 - Prob. 2.1KP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1. Calculate the number of iron atoms in a 1000kg of iron. 2. Calculate the volume in a cubic centi meters occupied by one mole of boron.arrow_forwardGallium has an orthorhombic structure,with a0 5 0.45258 nm, b0 5 0.45186 nm,and c0 5 0.76570 nm. The atomic radiusis 0.1218 nm. The density is 5.904 g/cm3,and the atomic weight is 69.72 g/mol.Determine(a) the number of atoms in each unit cell;and(b) the packing factor in the unit cell.arrow_forwardCalculate the gravitational constant g, in SI units, for alocation on the surface of the sun.arrow_forward
- Define the Atomic Packing Factor and explain its significance. If metal A has a higher APF than metal B, how do the properties of A compare to Barrow_forwardConsider the ReO3 unit cell shown below, with a = 3.74 Å and ∆ Hsub = 626 kj/mol (a) What is the Bravais lattice and smallest atomic basis that describes ReO3? Write the coordinates of each atom in the basis. (b) What is the coordination and bonding geometry for the Re and O atom?arrow_forwardIf the atomic radius of a metal that has the face centred cubic (FCC) crystal structure is (3.70x10^-1) nm, calculate the volume of its unit cell (in nm^3).arrow_forward
- electrochemistry. One form of TiO2 is the mineral rutile, which has a tetragonal lattice with a = b= 4.594 x 10-10 m and c = 2.959 x10-10 m at 25o C. There are two formula units per unit cell. Calculate the density of rutile at 25o Carrow_forwardChemistry Consider FCC copper. If bond energy per atom is 5.62 × 10-19 J, and its atomic radius is 0.128 nm, the surface energy for (100) plane will bearrow_forwardFor a face centered cubic (FCC) crustal structure, a total of ______ whole atoms can be found inside and FCC unit cellarrow_forward
- Zinc crystallizes in HCP structure. Geometrically the height of the unit cell is 43.4378 x10^-8 cm 4.34378x 10^-8 cm 2.1718 x10^-8cm data insufficientarrow_forwardGiven that molybdenum (Mo) with atomic weight 95.94 g/mol and density 10.2 g/cm3 assumes a body-centered cubic (BCC) crystal structure, estimate its atomic radius in pm.arrow_forwardAluminum metals when crystallizes form an FCC structure. If the edge of the unit cell is 353.56 pm, what is the diagonal length of each face of each unit cell?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning
Understanding Motor ControlsMechanical EngineeringISBN:9781337798686Author:Stephen L. HermanPublisher:Delmar Cengage Learning

Understanding Motor Controls
Mechanical Engineering
ISBN:9781337798686
Author:Stephen L. Herman
Publisher:Delmar Cengage Learning