
Concept explainers
Interpretation:
Fertilizers given in the problem statement has to be arranged in decreasing order of mass percentage of nitrogen.
Concept Introduction:
Relative amount of the elements present in a particular compound is same always. It does not matter where the compound is obtained or prepared. Mass percentage can be calculated by simple calculation using the mass of the element present in the compound and the total mass of the compound.
Explanation of Solution
(a)
Mass percentage of nitrogen in
Molecular formula of fertilizer is
Therefore, molecular mass of
Mass percentage of nitrogen:
Considering the atomic mass of nitrogen, and the molecular mass of
Therefore, mass percentage of nitrogen in
(b)
Mass percentage of nitrogen in
Molecular formula of fertilizer is
Therefore, molecular mass of
Mass percentage of nitrogen:
Considering the atomic mass of nitrogen, and the molecular mass of
Therefore, mass percentage of nitrogen in
(c)
Mass percentage of nitrogen in
Molecular formula of fertilizer is
Therefore, molecular mass of
Mass percentage of nitrogen:
Considering the atomic mass of nitrogen, and the molecular mass of
Therefore, mass percentage of nitrogen in
(d)
Mass percentage of nitrogen in
Molecular formula of fertilizer is
Therefore, molecular mass of
Mass percentage of nitrogen:
Considering the atomic mass of nitrogen, and the molecular mass of
Therefore, mass percentage of nitrogen in
(e)
Mass percentage of nitrogen in
Molecular formula of fertilizer is
Therefore, molecular mass of
Mass percentage of nitrogen:
Considering the atomic mass of nitrogen, and the molecular mass of
Therefore, mass percentage of nitrogen in
(f)
Mass percentage of nitrogen in
Molecular formula of fertilizer is
Therefore, molecular mass of
Mass percentage of nitrogen:
Considering the atomic mass of nitrogen, and the molecular mass of
Therefore, mass percentage of nitrogen in
The order of decreasing mass percentage of nitrogen in the given fertilizer can be depicted as shown below.
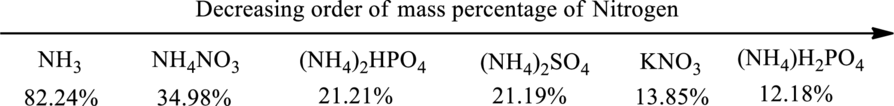
Want to see more full solutions like this?
Chapter 2 Solutions
GENERAL CHEMISTRY ACHIEVE ACCESS W/BOOK
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





