
Concept explainers
Interpretation: All the lone pairs for each of the following compounds should be drawn.
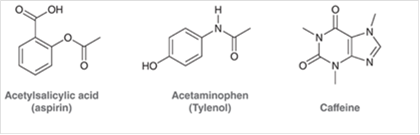
Concept Introduction The unshared pair of electrons are said to be lone pairs of electrons that are present in an atom of a compound.
Answer to Problem 34PP
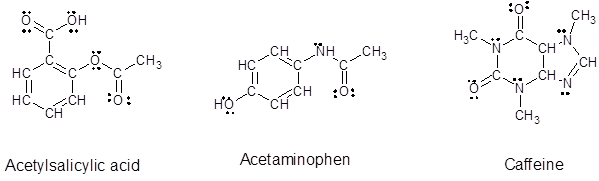
Explanation of Solution
The number of valence electrons in each isolated atom present in given compound are:
For Carbon = 4
For Hydrogen = 1
For Nitrogen = 5
For Oxygen = 6
Identify the number of bonds in the given compound:
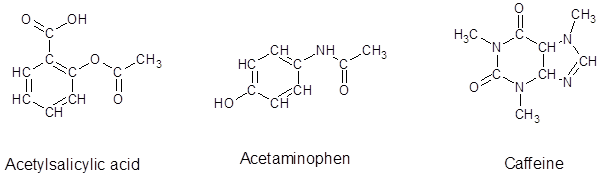
From the given structure of acetylsalicylic acid, each carbon atoms forms four bonds, hydrogen atoms forms one bond and oxygen atoms forms two bonds.
From the given structure of acetaminophen, each carbon atoms forms four bonds, hydrogen atoms forms one bond, oxygen atoms forms two bonds and nitrogen atom forms three bonds.
From the given structure of caffeine, each carbon atoms forms four bonds, hydrogen atoms forms one bond, oxygen atoms forms two bonds and nitrogen atom forms three bonds.
Since, all the valence electrons are involved in bond formation for carbon and hydrogen so there are no lone pair electrons on them. For all the oxygen atoms that forms two bonds uses two valence electrons out of six to form bond and rest four electrons are present as two lone-pair on each oxygen atom. For all the nitrogen atoms that forms three bonds uses three valence electrons out of five to form bond and rest two electrons are present as a lone-pair on each nitrogen atom. So, all the lone pairs for each compound are:
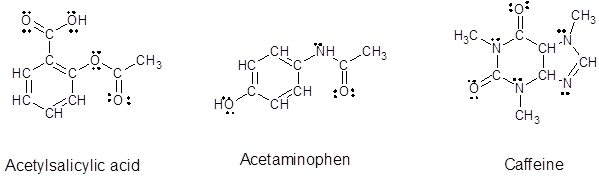
Want to see more full solutions like this?
Chapter 2 Solutions
ORGANIC CHEMISTRY,SOLNS...-ETEXT+BOX
- Draw in all the lone pairs on each structure.arrow_forwardDraw structural formulas for all constitutional isomers that have the given molecular formula: a) C2H6O and b) C3H7Narrow_forwardIdentify whether these organic molecules are constitutional isomers,resonance structures, the same molecule, or neither of the following,arrow_forward
- Please help me with this Q): name and draw structural formulas for all constitutional isomers of molecular formula C7H16arrow_forwardDraw the structure of the four constitutional isomers of molecular formula C 3H 6Br 2.arrow_forwardDraw 10 isomers (skeletal structure) with the formula C8H18arrow_forward
- Draw a three-D structure (entire molecule) for the following: a)CO2 b)SeF4arrow_forwardFor which compounds can a second resonance structure be drawn?Draw an additional resonance structure and the hybrid for eachresonance-stabilized compound.arrow_forwardDraw in all hydrogens and lone pairs on the charged carbons in each ion ?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





