
Concept explainers
Propranolol is an antihypertensive agent—that is, it lowers blood pressure. (a)
Which proton in propranolol is most acidic? (b) What products are formed when propranolol is treated with
(a)
Interpretation: The most acidic proton in propranolol is to be identified.
Concept introduction: An acid is a substance that is capable to donate
Answer to Problem 36P
The most acidic proton is
Explanation of Solution
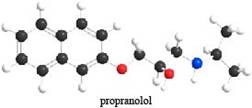
The given ball-and-stick model of propranolol is,
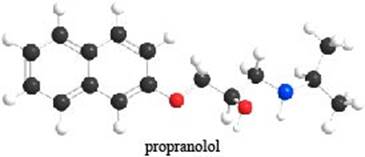
Figure 1
In ball-and-stick model, each colored ball represents a specific atom and each stick represents a bond. In this model, each black ball represents
Thus, the given compound is drawn as,
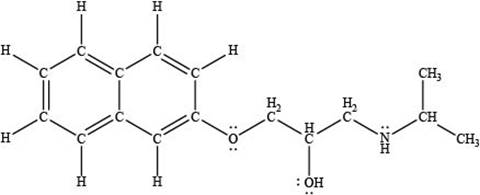
Figure 2
The acidity of an acid is directly proportional to the resonance stabilization of its conjugate base. In propranolol, when the
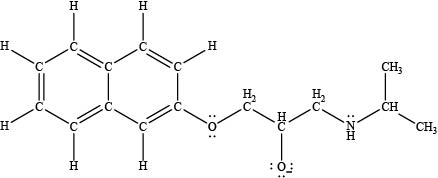
Figure 3
Thus, the most acidic proton is
The most acidic proton is
(b)
Interpretation: The products formed by the treatment of propranolol with
Concept introduction: Sodium hydride
Answer to Problem 36P
The product formed by the treatment of propranolol with
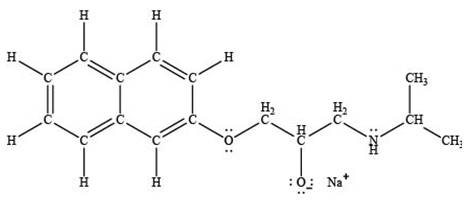
Explanation of Solution
Sodium hydride
The products formed by the treatment of propranolol with
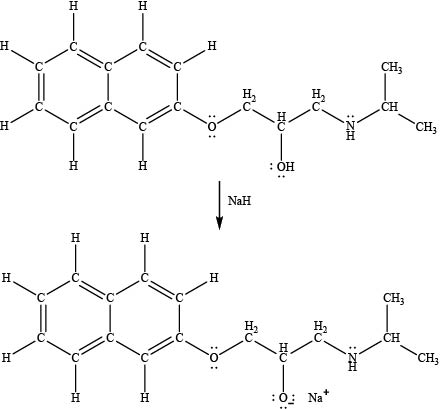
Figure 4
The product formed by the treatment of propranolol with
(c)
Interpretation: The most basic atom in propranolol is to be identified.
Concept introduction: A base is a substance that is capable to accept a
Answer to Problem 36P
The most basic atom is
Explanation of Solution
A base is a substance that is capable to accept
Thus, the most basic atom is
The most basic atom is N of
(d)
Interpretation: The products formed by the treatment of propranolol with
Concept introduction: Hydrogen chloride
Answer to Problem 36P
The product formed by the treatment of propranolol with
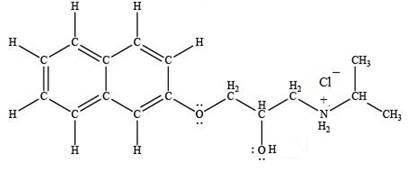
Explanation of Solution
Hydrogen chloride
The product formed by the treatment of propranolol with
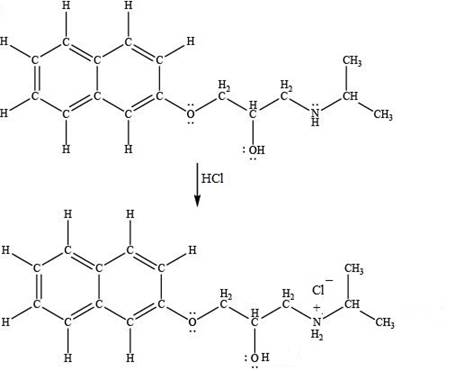
Figure 5
The product formed by the treatment of propranolol with
Want to see more full solutions like this?
Chapter 2 Solutions
Package: Loose Leaf For Organic Chemistry With Connect Access Card (1 Semester)
- Rank the labeled N atoms in the anticancer drug imatinib (trade nameGleevec) in order of increasing basicity. Imatinib, sold as a salt withmethanesulfonic acid (CH3SO3H), is used for the treatment of chronicmyeloid leukemia as well as certain gastrointestinal tumors.arrow_forwardPropoxide (CH3CH2CH2O- ) is a larger molecule than ethoxide (CH3CH2O- ), yet they are equally basic. Explain why they are equally basic.arrow_forwardRank and explain the following compounds in order of increasing acidityarrow_forward
- 2-Hydroxybutanedioic acid occurs naturally in apples and other fruits. Rank the labeled protons (Ha−He) in order of increasing acidity, and explain in detail the order you chose.arrow_forwardWhich compound has the higher acidity? Explain.arrow_forwardRank the following compounds in order increasing basic strength.arrow_forward
- please explain and rank the following compound in order of increasing acidityarrow_forwardWhich of the attached anions is the stronger base? Explain your choice.arrow_forwardCiprofloxacin is a member of the fluoroquinolone class of antibiotics.Which of the N atoms is the most basic? Explainarrow_forward
