
Concept explainers
(a)
To determine: Sketch of a labeled voltaic cell and the concentration of the solution.
(a)
Answer to Problem 1DE
The labeled sketch of the voltaic cell is,
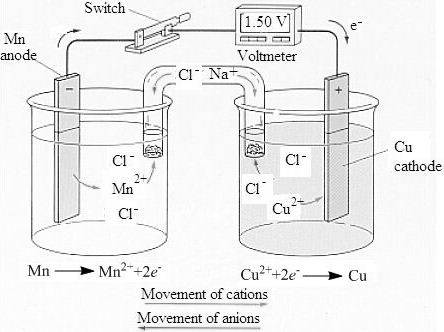
The concentration of the copper and the manganese ion is
Explanation of Solution
Given
The electrical potential output of the cell is
The external device draws the current of
The electric potential of the cell is calculated as,
According to the above equation, the difference in the oxidation and the reduction potential should be
The standard oxidation potential of manganese is
Therefore, the pair of manganese and copper is used to construct a voltaic cell with manganese being anode at which oxidation takes place and copper being cathode at which reduction takes place.
The standard reduction potential is the potential of the electrode dipped in the aqueous solution of its ion of concentration
Therefore, the concentration of the copper and the manganese ion is
We are given the beaker of the capacity of
The volume of the solution of manganese and copper ion is assumed to be
The labeled sketch of the voltaic cell is,
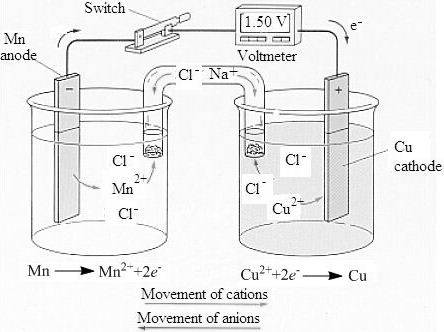
- Figure. 1
The mass of the manganese in the
Substitute the value of molar concentration, molar mass and the volume of the solution of manganese in the above equation.
The mass of the copper in the
Substitute the value of molar concentration, molar mass and the volume of the solution of copper in the above equation.
Therefore, the mass of manganese and copper present in the
The concentration of the copper and the manganese ion is
(b)
To determine: The concentration of manganese and copper ion in the solution after
(b)
Answer to Problem 1DE
Explanation of Solution
Given
The electrical potential output of the cell is
The external device draws the current of
The charge transport is calculated by the formula,
Substitute the value of current and time in the above formula,
Charge on each electron is
The moles of electron present in
Substitute the value of charge transfer, charge on each electron and Avogadro’s number in the above formula.
Two electron transfer takes place by oxidation and reduction of manganese and copper, respectively.
Therefore, the change in moles of manganese and copper is:
The initial concentration of the ions is
Thus, one mole of ion is present in one liter of the solution and
The number of moles of manganese ion increase due to oxidation of metal. Therefore, the molar concentration of manganese ion in
The number of moles of copper ion increase due to oxidation of metal. Therefore, the molar concentration of copper ion in
Thus, the concentration of manganese and copper ion after two hours is
The concentration of manganese and copper ion after two hours is
(c)
To determine: The voltage that a cell register at the end of the discharge.
(c)
Answer to Problem 1DE
Explanation of Solution
Given
The electrical potential output of the cell is
The external device draws the current of
The concentration of manganese and copper ion after two hours is
The standard oxidation potential of manganese is
The standard reduction potential is the potential of the electrode dipped in the aqueous solution of its ion of concentration
Thus, one mole of manganese ion gives the oxidation potential of
Therefore,
One mole of copper ion gives the reduction potential of
Therefore,
The electric potential of the cell at discharge is calculated as,
Substitute the value of oxidation and reduction potential in the above equation.
Thus, the end cell potential is
The end cell potential is
(d)
To determine: The time taken for the reactant of one cell to get completely consumed.
(d)
Answer to Problem 1DE
Explanation of Solution
Given
The electrical potential output of the cell is
The external device draws the current of
The charge transport is calculated by the formula,
Substitute the value of current and time in the above formula,
Charge on each electron is
The moles of electron present in
Substitute the value of charge transfer, charge on each electron and Avogadro’s number in the above formula.
Two electron transfer takes place by oxidation and reduction of manganese and copper, respectively.
Therefore, the change in moles of manganese and copper is
Charge is directly proportional to time at constant current flow.
The charge transferred is
The total initial concentration of the reactant of one half cell is one molar.
Therefore, the charge transfer of one molar takes place in
The charge transfer of one molar takes place in
Want to see more full solutions like this?
Chapter 20 Solutions
CHEMISTRY THE CENTRAL SCIENCE 14TH EDI
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





