
Concept explainers
Interpretation: The basic
Concept introduction: Deoxyribose nucleic acid is the basic functional unit of a cell. It consists of deoxyribose sugar, phosphate group and four nucleic bases. The
To determine: The basic functional groups in the given structures.
Answer to Problem 20.1VP
Solution
The functional group present in adenine is primary amine, in guanine is primary and secondary amine, in thymine is two secondary amine and in cytosine is primary and secondary
Explanation of Solution
Explanation
Adenine is a
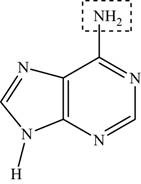
Figure 1
The functional group present on guanine is amine
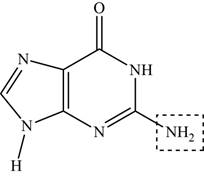
Figure 2
Thymine consists of two secondary amine
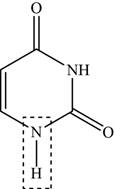
Figure 3
The functional groups attached to cytosine are one primary amine, two secondary amines, and one carbonyl group as shown in Figure 4.
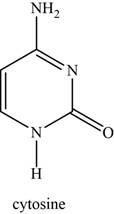
Figure 4
Conclusion
The functional group present in adenine is primary amine, in guanine is primary and secondary amine, in thymine is two secondary amine and in cytosine is primary and secondary amines along with carbonyl group
Want to see more full solutions like this?
Chapter 20 Solutions
CHEMISTRY:SCI.IN CONTEXT (CL)-PACKAGE
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





