
Concept explainers
(a)
Interpretation:
On the periodic table, the position of the given element has to be located.
(a)
Explanation of Solution
Given electronic configuration,
Electron filling concepts in orbitals:
- Electrons first occupy the orbitals with lower energy than the orbitals with higher energy (Aufbau principle).
- An orbital can be occupied only by two electrons having opposite spins (Pauli exclusion principle).
- Each electron fills each orbital till it is half filled, when they are degenerate orbital (Hund’s rule).
The order of electrons filling in a multi-electron atom is given as follows,
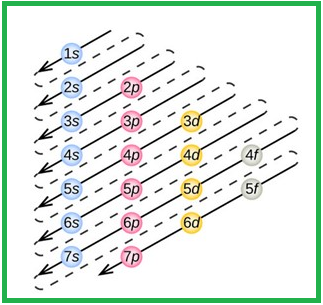
Figure 1
The order of orbitals in their increasing energy is given by
Calculate the total number of electrons in the given electronic configuration and identify the element
The position of the given element on the periodic table is given below:
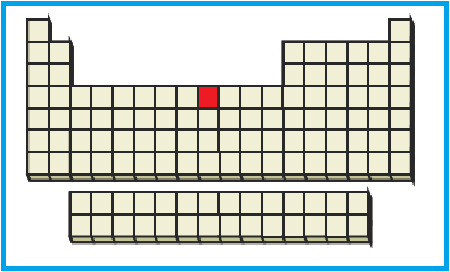
Figure 2
(b)
Interpretation:
On the periodic table, the position of the given element has to be located.
(b)
Explanation of Solution
Given electronic configuration,
Electron filling concepts in orbitals:
- Electrons first occupy the orbitals with lower energy than the orbitals with higher energy (Aufbau principle).
- An orbital can be occupied only by two electrons having opposite spins (Pauli exclusion principle).
- Each electron fills each orbital till it is half filled, when they are degenerate orbital (Hund’s rule).
The order of electrons filling in a multi-electron atom is given as follows,

Figure 1
The order of orbitals in their increasing energy is given by
Calculate the total number of electrons in the given electronic configuration and identify the element
The position of the given element on the periodic table is given below:
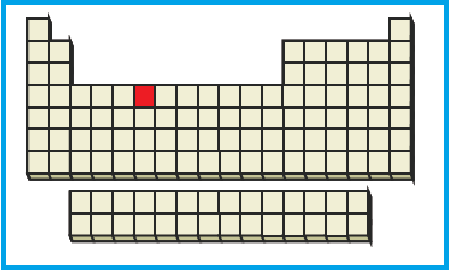
Figure 3
(c)
Interpretation:
On the periodic table, the position of the given element has to be located.
(c)
Explanation of Solution
Given electronic configuration,
Electron filling concepts in orbitals:
- Electrons first occupy the orbitals with lower energy than the orbitals with higher energy (Aufbau principle).
- An orbital can be occupied only by two electrons having opposite spins (Pauli exclusion principle).
- Each electron fills each orbital till it is half filled, when they are degenerate orbital (Hund’s rule).
The order of electrons filling in a multielectron atom is given as follows,

Figure 1
The order of orbitals in their increasing energy is given by
Calculate the total number of electrons in the given electronic configuration and identify the element
The position of the given element on the periodic table is given below:
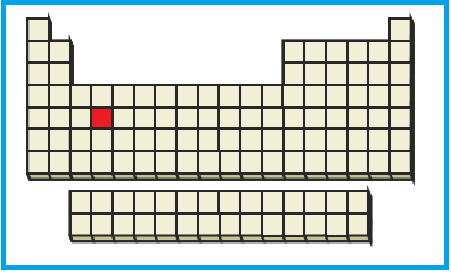
Figure 4
(d)
Interpretation:
On the periodic table, the position of the given element has to be located.
(d)
Explanation of Solution
Given electronic configuration,
Electron filling concepts in orbitals:
- Electrons first occupy the orbitals with lower energy than the orbitals with higher energy (Aufbau principle).
- An orbital can be occupied only by two electrons having opposite spins (Pauli exclusion principle).
- Each electron fills each orbital till it is half filled, when they are degenerate orbital (Hund’s rule).
The order of electrons filling in a multielectron atom is given as follows,

Figure 1
The order of orbitals in their increasing energy is given by
Calculate the total number of electrons in the given electronic configuration and identify the element
The position of the given element on the periodic table is given below:
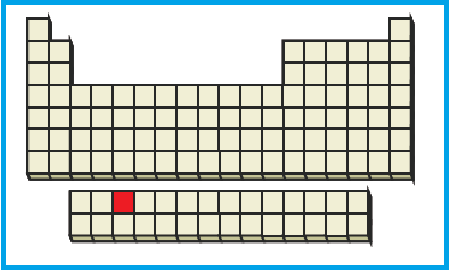
Figure 5
Want to see more full solutions like this?
Chapter 20 Solutions
General Chemistry: Atoms First, Books a la Carte Edition; Modified Mastering Chemistry with Pearson eText -- ValuePack Access Card -- for General Chemistry: Atoms First (2nd Edition)
- Four different octahedral chromium coordination compounds exist that all have the same oxidation state for chromium and have H2O and Cl as the ligands and counterions. When 1 mole of each of the four compounds is dissolved in water, how many moles of silver chloride will precipitate upon addition of excess AgNO3?arrow_forwardWhat is the coordination number of the central metal atom in the following complexes? (a) [Fe(H2O)63+] (b) [Pt(NH3)Br3] (c) [V(en)Cl42] (d) [Au(CN)2+]arrow_forwardAn aqueous solution of [Rh(C2O4)3]3− is yellow. Predict the approximate wavelength and predominant color of light absorbed by the complex.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





