
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
9th Edition
ISBN: 9780137249442
Author: Wade
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 20.34SP
Given the structure of ascorbic acid (vitamin C):
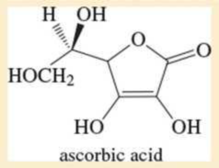
- a. Is ascorbic acid a carboxylic acid?
- b. Compare the acid strength of ascorbic acid (pKa = 4.71) with acetic acid.
- c. Predict which proton in ascorbic acid is the most acidic.
- d. Draw the form of ascorbic acid that is present in the body (aqueous solution, pH = 7.4).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
61. Give the products of the following acid-base reactions and indicate whether reactants or products are favored at equilibrium. (Use the pk, values that are
given in Section 2.3.)
CH,NH; =
a. CH,COH + CH;0
b. CH,CH,OH + NH,
c. CH;COH +
d. CH;CH,OH + HCI =
This reaction makes a carboxylic
acid, draw the reaction peoducts
for both 1 and 2
bazaupalb an aolu DA
1. KMnO4, NaOH,
H₂O, heat
2. H₂O, H3O+ catalyst
Amino acids such as glycine are the building blocks of large molecule.called proteins that give structure to muscle, tendon, hair, and nails.a.Explain why glycine does not actually exist in the form with all atoms uncharged, but actually exists as a salt called a zwitterion.
b.What product is formed when glycine is treated with concentrated HCl?
c. What product is formed when glycine is treated with NaOH?
Chapter 20 Solutions
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
Ch. 20.2C - Prob. 20.1PCh. 20.2C - Name the following carboxylic acids (when...Ch. 20.4B - Rank the compounds in each set in order of...Ch. 20.5 - Prob. 20.4PCh. 20.5 - Phenols are less acidic than carboxylic acids,...Ch. 20.5 - Prob. 20.6PCh. 20.7A - Prob. 20.7PCh. 20.7B - Prob. 20.8PCh. 20.7D - Draw all four resonance forms of the fragment at...Ch. 20.7D - a. Why do most long-chain fatty acids show a large...
Ch. 20.10 - Prob. 20.13PCh. 20.10 - A carboxylic acid has two oxygen atoms, each with...Ch. 20.10 - Prob. 20.15PCh. 20.10 - The mechanism of the Fischer esterification was...Ch. 20.10 - Prob. 20.17PCh. 20.12 - Show how to synthesize the following compounds,...Ch. 20.13 - Show how you would synthesize the following...Ch. 20.14 - Prob. 20.20PCh. 20.14 - Prob. 20.21PCh. 20.15 - Propose a mechanism for the reaction of benzoic...Ch. 20.15 - Prob. 20.23PCh. 20.15 - Prob. 20.24PCh. 20 - Prob. 20.25SPCh. 20 - Give both IUPAC names and common names for the...Ch. 20 - Draw the structures of the following compounds. a....Ch. 20 - Prob. 20.28SPCh. 20 - Arrange each group of compounds in order of...Ch. 20 - Predict the products (if any) of the following...Ch. 20 - Rank the following isomers in order of increasing...Ch. 20 - Prob. 20.32SPCh. 20 - What do the following pKa values tell you about...Ch. 20 - Given the structure of ascorbic acid (vitamin C):...Ch. 20 - Prob. 20.35SPCh. 20 - Show how you would accomplish the following...Ch. 20 - Predict the products and propose mechanisms for...Ch. 20 - Prob. 20.38SPCh. 20 - Prob. 20.39SPCh. 20 - Prob. 20.40SPCh. 20 - Prob. 20.44SPCh. 20 - Prob. 20.45SPCh. 20 - Predict the major form of each compound when it is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- C. d. لله CI OEt OCH3 1 eq Me₂NH Pyridine 1. PhCO₂Me NaOEt EtOH 2. aq H* 1. LAH 2. H₂O aq H+ A H₂SO4 EtOHarrow_forwardWhich of the following molecules is the most acidic? A. 2,2-diphenylacetic acid B. trichloroacetic acid C. acetic acid D. trifluoroacetic acid E. 2,2-dimethylacetic acidarrow_forwardThe Ka1 of ascorbic acid is 7.94 x 10-5. Would you expect ascorbic acid dissolved in blood plasma (pH 7.35–7.45) to exist primarily as ascorbic acid or as ascorbate anion? Explain.arrow_forward
- Draw the products of each acid-base reaction. NaOH b. + NazCO3 a. + CH3CH,CH, HO, OHarrow_forwardWhat is the role of phenolphthalein in the neutralization reaction? Draw the structure of phenolphthalein under acidic and basic conditions.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. I I I I Mg. :O: Br. :O: Select to Add Arrows :O: CO2 THE H Mg 0: Br Please select a drawing or reagent from the question areaarrow_forward
- 15. Which of the following molecules is acetic acid? а. СН:СООН b. CH3COONa c. CH3CH2COOH d. CH3CH2COONAarrow_forwardWhich of the following molecules is the most acidic? A. 3-chloroacetic acid B. 2-chloroacetic acid C. trichloroacetic acid D. trifluoroacetic acid E. triliodoacetic acidarrow_forwardCan someone help answer these questions please? Thank you in advance.arrow_forward
- If an amino acid is in a acidic solution, which form does the carboxyl group take? a. —CO2– b. —CO2H+ c. —CO2– d. —CO2Harrow_forwardWhich set of answers is organized in orderfrom highest to lowest priority?A. –CH2NH2 > –CN > –COOH > –NH2B. –COOH > –CN > –CH2NH2 > –NH2C. –NH2 > –CN > –CH2NH2 > –COOHD. –NH2 > –COOH > –CN > –CH2NH2E. –CN > –NH2 > –CH2NH2 > –COOHarrow_forwardDraw the following reactions and label it properly 1. stearic acid reaction with water chemical equation 2. Reaction with NaOH with benzoic acid 3. Reaction with NaOH with acetic acid 4. Reaction with NaOH with stearic acid 5. Reaction with sodium carbonate with acetic acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY