
Concept explainers
(a)
Interpretation:
To determine the magnetic property of the given compounds.
Concept Introduction:
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
To Identify: To determine the magnetic property of the given compounds.
(a)
Answer to Problem 20.64SP
The
Explanation of Solution
Interpret the given information.
The
(b)
Interpretation:
To determine the magnetic property of the given compounds.
Concept Introduction:
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
To Identify: To determine the magnetic property of the given compounds.
(b)
Answer to Problem 20.64SP
The systematic name of the given compound
Explanation of Solution
Interpret the given information.
The systematic name of the given compound
The six ligands around the metal ion give octahedral geometry.
Magnetic property of the compound:
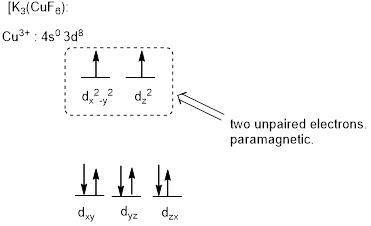
The high field complex ion possesses eight d-electrons and the two electrons are unpaired and exhibits paramagnetic.
(c)
Interpretation:
To determine the magnetic property of the given compounds.
Concept Introduction:
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
To Identify: To determine the magnetic property of the given compounds.
(c)
Answer to Problem 20.64SP
The square-planar geometry will be diamagnetic in nature.
Explanation of Solution
Interpret the given information.
In complex ion of
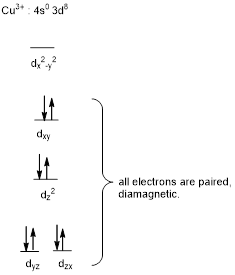
In complex ion of
Want to see more full solutions like this?
Chapter 20 Solutions
GENERAL CHEMISTRY >IC<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





