
Concept explainers
Thioesters
Thioesters have the general formula 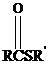 . They resemble their oxygen counterparts
. They resemble their oxygen counterparts 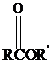
(oxoesters) in structure and reactivity more than other carboxylic acid derivatives such as acyl
chlorides, acid anhydrides, and amides. Thioesters can be prepared from thiols by reaction with acyl chlorides or acid anhydrides in much the same way as oxoesters are prepared from
alcohols.
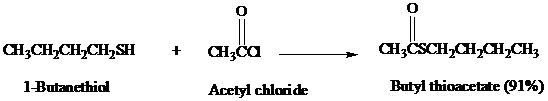
The preparation of thioesters by Fischer esterification is not very effective, however, because
the equilibrium is normally unfavorable. Under conditions in which ethanol is converted to ethyl
benzoate to the extent of
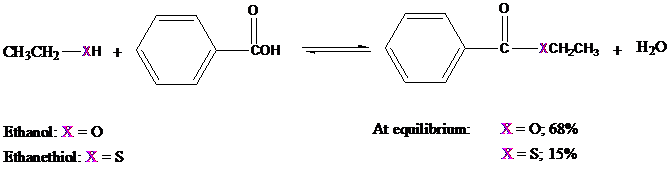
This, and numerous other observations, indicates that
Like chlorine, sulfur is a third-row element and does not act as an electron-pair donor to the carbonyl group as well as oxygen.
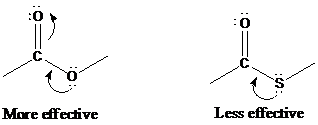
Thioesters and oxoesters are similar in their rates of nucleophilic acyl substitution, except with
amine nucleophiles for which thioesters are much more reactive. Many biological reactions involve nucleophilic acyl substitutions referred to as acyl transfer reactions. The thioester acetyl coenzyme A is an acetyl group donor to alcohols,
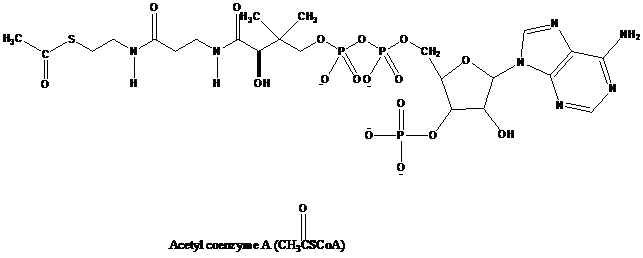 Melatonin, a hormone secreted by the pineal gland that regulates circadian rhythms, including wake–sleep cycles, is biosynthesized by a process in which the first step is an enzyme-catalyzed transfer of the acetyl group from sulfur of acetyl coenzyme A to the
Melatonin, a hormone secreted by the pineal gland that regulates circadian rhythms, including wake–sleep cycles, is biosynthesized by a process in which the first step is an enzyme-catalyzed transfer of the acetyl group from sulfur of acetyl coenzyme A to the

Acetylcholine is a neurotransmitter formed in nerve cells by the enzyme-catalyzed reaction
of choline with acetyl coenzyme A.
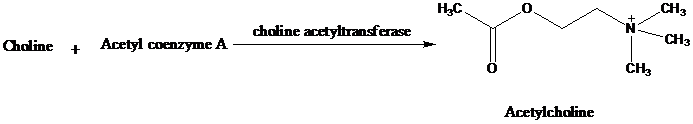
What is the most reasonable structure for choline?
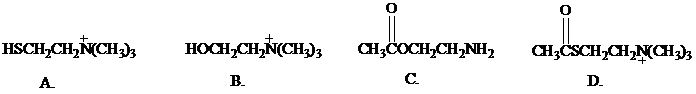
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Explain the solubility behavior of each compound in water with relevance to their structure. a. Acyl compounds in water-acetic acid (clear colorless solution)-benzoic acid (turbid, cloudy solution)-sodium benzoate (clear colorless solution)arrow_forwardProvide an explanation (in detail) on how base catalysts speed up nucleophilic reactions of carbonyl compounds.arrow_forwardThe reaction of a nitrile with an alcohol in the presence of a strong acid forms an N-substituted amide. This reaction, known as the Ritter reaction, doesnot work with primary alcohols. a. Why does the Ritter reaction not work with primary alcohols? b. Provide an explanation for why an amide is less susceptible to nucleophilic attack than its corresponding ester.arrow_forward
- Define what is reduction reaction, reducing agents, weak and strong reducing agents sodium borohydride reduction with benzophenone and sodium borohyride to get benzophenonearrow_forwardRank the derivatives of benzoic in order of increasing acidity:arrow_forwardPrimary amines can also be prepared by the reaction of an alkyl halide with azide ion, followed by catalytic hydrogenation. What advantage do this method and the Gabriel synthesis have over the synthesis of a primaryamine using an alkyl halide and ammonia?arrow_forward
- Why is it not advisable to use aqueous hydrochloric acid in a Grignard reaction of a ketone? A) The Grignard reagent will react with the acid and cannot react with the ketone. B) The ketone will be protonated and will become unreactive. C) The ketone will form an unreactive enol. D) The Grignard reagent won't dissolve in aqueous solutionsarrow_forwardArrange the compounds in order of INCREASING reactivity towards bromination. Toluene, Nitrobenzene, Anisole, Aniline Acetophenone, Bromobenzene, Aniline, Phenol Acetanilide, Benzaldehyde, Toluene, Iodobenzenearrow_forwardRank the following carboxylic acid derivatives in decreasing order (most to least) of reactivity towards nucleophilic acyl substitution.arrow_forward
- Both pyridine and pyrrole are nitrogen containing aromatic heterocyclic compounds. When treated with HCl, only pyridine forms the hydrochloride salt, where as pyrrole is unreactive. What is the best explanation for this observed reactivity.arrow_forwardTyramine is an alkaloid found in mistletoe and ripe cheese. Dopamine is a neurotransmitter involved in the regulation of the central nervous system. a. How can tyramine be prepared from β-phenylethylamine?b. How can dopamine be prepared from tyramine?c. Give two ways to prepare β-phenylethylamine from β-phenylethyl chloride.d. How can β-phenylethylamine be prepared from benzyl chloride?e. How can β-phenylethylamine be prepared from benzaldehyde?arrow_forwardWhat two amides are obtained from the reaction of acetyl chloride with an equivalent of ethylamine and an equivalent of propylamine? a. Why is only one amide obtained from the reaction of acetyl chloride with an equivalent of ethylamine and an equivalent of triethylamine?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


