
ORGANIC CHEM LOOSE LEAF W/ FREE SOLUTI
9th Edition
ISBN: 9780134588902
Author: Wade
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20.10, Problem 20.16P
The mechanism of the Fischer esterification was controversial until 1938, when Irving Roberts and Harold Urey of Columbia University used isotopic labeling to follow the alcohol oxygen atom through the reaction. A catalytic amount of sulfuric acid was added to a mixture of 1 mole of acetic acid and 1 mole of special methanol containing the heavy 18O isotope of oxygen. After a short period, the acid was neutralized to stop the reaction, and the components of the mixture were separated.
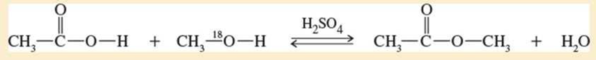
- a. Propose a mechanism for this reaction.
- b. Follow the labeled 18O atom through your mechanism, and show where it is found in the products.
- c. The 18O isotope is not radioactive. Suggest how you could experimentally determine the amounts of 18O in the separated components of the mixture.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Rate Enhancement by Urease.The enzyme urease enhances the rate of urea hydrolysis at pH 8.0 and 20 °C by a factor of 1014. If a given quantity of urease can completely hydrolyze a given quantity of urea in 5.0 min at 20 °C and pH 8.0, how long would it take for this amount of urea to be hydrolyzed under the same conditions in the absence of urease? Assume that both reactions take place in sterile systems so that bacteria cannot attack the urea.
Organic chemistry 2 :write the complete mechanism for the formation of benzalacetone .
How was Thin Layer Chromatography (TLC) used in organic synthesis reactions ?
TLC is used to find the exact concentration of a catalyst in the reaction.
TLC can speed up a reaction because silica gel is polar.
TLC can separate components of a reaction mixture such as starting materials
and products allowing the experimenter to identify what compounds are in the
reaction mixture.
TLC is used to find percentage yield of the reaction.
Chapter 20 Solutions
ORGANIC CHEM LOOSE LEAF W/ FREE SOLUTI
Ch. 20.2C - Prob. 20.1PCh. 20.2C - Name the following carboxylic acids (when...Ch. 20.4B - Rank the compounds in each set in order of...Ch. 20.5 - Prob. 20.4PCh. 20.5 - Phenols are less acidic than carboxylic acids,...Ch. 20.5 - Prob. 20.6PCh. 20.7A - Prob. 20.7PCh. 20.7B - Prob. 20.8PCh. 20.7D - Draw all four resonance forms of the fragment at...Ch. 20.7D - a. Why do most long-chain fatty acids show a large...
Ch. 20.10 - Prob. 20.13PCh. 20.10 - A carboxylic acid has two oxygen atoms, each with...Ch. 20.10 - Prob. 20.15PCh. 20.10 - The mechanism of the Fischer esterification was...Ch. 20.10 - Prob. 20.17PCh. 20.12 - Show how to synthesize the following compounds,...Ch. 20.13 - Show how you would synthesize the following...Ch. 20.14 - Prob. 20.20PCh. 20.14 - Prob. 20.21PCh. 20.15 - Propose a mechanism for the reaction of benzoic...Ch. 20.15 - Prob. 20.23PCh. 20.15 - Prob. 20.24PCh. 20 - Prob. 20.25SPCh. 20 - Give both IUPAC names and common names for the...Ch. 20 - Draw the structures of the following compounds. a....Ch. 20 - Prob. 20.28SPCh. 20 - Arrange each group of compounds in order of...Ch. 20 - Predict the products (if any) of the following...Ch. 20 - Rank the following isomers in order of increasing...Ch. 20 - Prob. 20.32SPCh. 20 - What do the following pKa values tell you about...Ch. 20 - Given the structure of ascorbic acid (vitamin C):...Ch. 20 - Prob. 20.35SPCh. 20 - Show how you would accomplish the following...Ch. 20 - Predict the products and propose mechanisms for...Ch. 20 - Prob. 20.38SPCh. 20 - Prob. 20.39SPCh. 20 - Prob. 20.40SPCh. 20 - Prob. 20.44SPCh. 20 - Prob. 20.45SPCh. 20 - Predict the major form of each compound when it is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what is the role of NaOH in this reaction mechanism?arrow_forwardThis is the type of organic reaction wherein a single reactant splits into two products and is accompanied by the formation of a small molecule. addition substitution elimination condensation What is the name of the angle that a nucleophile approaches the carbon on the C=O bond? Borge-Dante Bürgi–Dunitz Baran-Daly Bunsen-Debyearrow_forwardTrimethylamine, (CH3)3N, is a common reagent. It interacts readily with diborane gas, B₂H6. The latter dissociates to BH3 and this forms a complex with the amine, (CH3)3N→BH3. The reaction between trimethylamine and borane is shown. CH3 CH3 H CH3-N : + B-H CH3-N B-H CH3 H CH3 H Is the BH3 fragment a Lewis acid or a Lewis base? To decide this, answer the following questions: H a Which of the following is a definition of a Lewis acid? proton donor electron pair acceptor proton acceptor electron pair donorarrow_forward
- Write the reaction flow chart fir the following condensation and further heating of resulting productsarrow_forwardPleasearrow_forwardConsider the reaction below and answer the question below the reaction. A student performed a synthesis of ethyl 4-aminobenzoate (C) as shown in the reaction above. The student added 1.20 g of 4-aminobenzoic acid and 12.0 mL of ethanol together in the presence of sulfuric acid. The reaction mixture was heated to reflux for a period of 45 minutes. H₂N OH + CH3CH₂OH ethanol ethanol MM 46.10 g/mol 4-aminobenozoate sulfuric acid H+ 4-aminobenzoic acid H₂N 4-aminobenzoic acid MM 137.14 g/m ol A B In the catalyzed reaction, which of the following is used as a catalyst? OCH₂CH3 + H₂O ethyl 4-aminobenzoate MM 165.19 g/mol сarrow_forward
- The enzyme urease increases the rate of urea hydrolysis at pH 8.0 and 20 °C by a factor of 1014. Suppose that a given quantity of urease can completely hydrolyze a given quantity of urea in 19 minutes at pH 8.0 and 20 °C. How long would it take for this amount of urea to be hydrolyzed in the absence of urease at the same temperature and pH in sterile conditions? Include two significant figures in your answer. timeuncatalyzed years * TOOLS x10arrow_forwardConsider this reaction: Br CH3OH Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. H. Br + CH;OH Br Intermediate 2 (product) Intermediate 1 In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixturearrow_forwardAt 25°C, for benzoate ion Kp = 1.54 x 10-10 while for ethanoate ion K = 5.5 x 10-10. Choose the correct answer: Select one: Ethanoate ion is a stronger base than benzoate ion but ethanoic acid is a weaker acid than benzoic acid. Ethanoate ion is a stronger base than benzoate ion but ethanoic acid is a stronger acid than benzoic acid. Ethanoate ion is a weaker base than benzoate ion and ethanoic acid is a weaker acid than benzoic acid. Ethanoate ion is a weaker base than benzoate ion but ethanoic acid is a stronger acid than benzoic acid.arrow_forward
- Consider the given reaction in which NC−NC− is the nucleophile and CH3CNCH3CN is the solvent. The reactant molecule has a structure with solid and dashed wedge bonds. A solid wedge () is used to show the bond that is above the plane of the paper, and a dashed wedge () is used to show the bond that is behind the plane of the paper. Draw the product of the following reaction:arrow_forwardThe reaction of the alkene, ethylene, with H2 produces ethane as a product. However, the reaction is incredibly slow in the absence of a catalyst, such as platinum metal. What is the role of the catalyst in speeding up the reaction? Raising the activation energy by breaking the bonds of hydrogen molecules. By bringing together the hydrogen atoms and alkene on the same metal surface, thereby lowering the activation energy. By decreasing the number of reactive inermediates. By increasing the equilibrium constant for the reaction.arrow_forwardConsider this reaction: Br CH;OH Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. Br + CH3OH Br Intermediate 1 Intermediate 2 (product) In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixturearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License