
Concept explainers
Acid-catalyzed hydrolysis of a nitrile to give a carboxylic acid occurs by initial protonation of the nitrogen atom, followed by nucleophilic addition of water. Review the mechanism of base-catalyzed nitrile hydrolysis in Section 20-7 and then predict the products for each reaction below and write all of the steps involved in the acid-catalyzed reaction, using curved arrows to represent electron flow in each step.
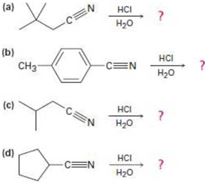
a)
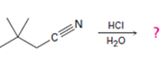
Interpretation:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step, is to be given.
Concept introduction:
The acid protonates the nitrogen of the nitrile group initially. The nucleophilic attack by water and subsequent proton transfer will yield an intermediate. Another nucleophilic attack by water on the intermediate and yet another proton transfer produces another intermediate. The intermediate loses ammonia to produce the protonated acid which deprotonates to yield the acid.
To give:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step.
Answer to Problem 25MP
The products of the reaction are ammonia and 2,2-dimethylbutanoic acid.
The mechanism of the reaction is given below.
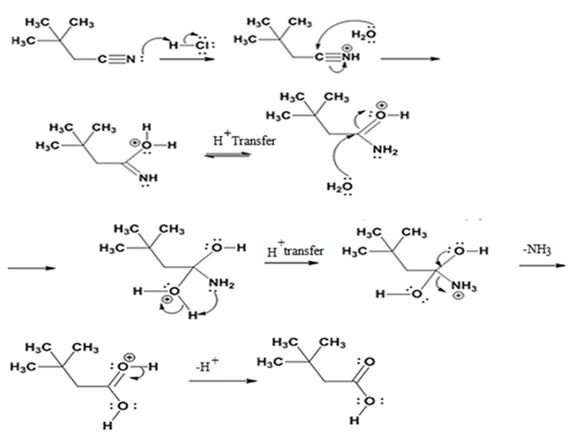
Explanation of Solution
In the first step, 2,2-dimethylbutane nitrile is protonated by HCl. In the next step, the nucleophilic attack of water on the protonated nitrile occurs and the accompanying proton transfer yields a protonated aminoketone. Another nucleophilic attack on the carbonyl carbon of the protonated aminoketone in the next step and the subsequent proton transfer yields a protonated diol intermediate which eliminates ammonia and a proton in the subsequent steps to yield 2,2-dimethylbutanoic acid.
The products of the reaction are ammonia and 2,2-dimethylbutanoic acid.
The mechanism of the reaction is given below.

b)
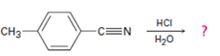
Interpretation:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step, is to be given.
Concept introduction:
The acid protonates the nitrogen of the nitrile group initially. The nucleophilic attack by water and subsequent proton transfer will yield an intermediate. Another nucleophilic attack by water on the intermediate and yet another proton transfer produces another intermediate. The intermediate loses ammonia to produce the protonated acid which deprotonates to yield the acid.
To give:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step.
Answer to Problem 25MP
The products of the reaction are ammonia and p-methylbenzoic acid.
The mechanism of the reaction is given below.
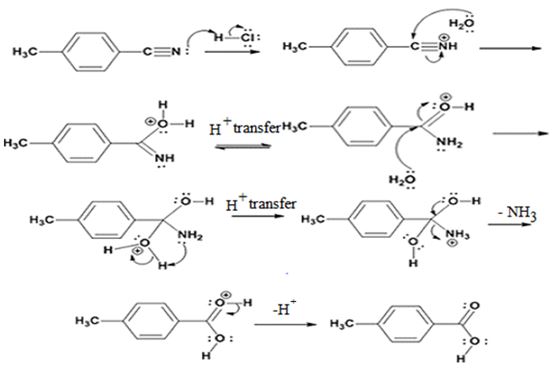
Explanation of Solution
In the first step, p-methylbenzonitrile is protonated by HCl. In the next step, the nucleophilic attack of water on the protonated nitrile occurs and the accompanying proton transfer yields a protonated aminoketone. Another nucleophilic attack on the carbonyl carbon of the protonated aminoketone in the next step and the subsequent proton transfer yields a protonated diol intermediate which eliminates ammonia and a proton in the subsequent steps to yield p-methylbenzoic acid.
The products of the reaction are ammonia and p-methylbenzoic acid.
The mechanism of the reaction is given below.

c)
Interpretation:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step, is to be given.
Concept introduction:
The acid protonates the nitrogen of the nitrile group initially. The nucleophilic attack by water and subsequent proton transfer will yield an intermediate. Another nucleophilic attack by water on the intermediate and yet another proton transfer produces another intermediate. The intermediate loses ammonia to produce the protonated acid which deprotonates to yield the acid.
To give:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step.
Answer to Problem 25MP
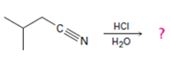
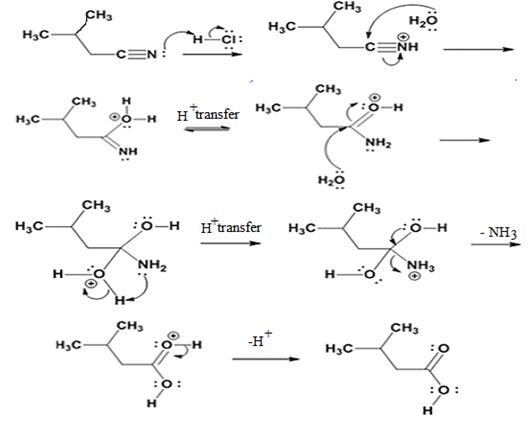
The products of the reaction are ammonia and 2-methylbutanoic acid.
The mechanism of the reaction is given below.
Explanation of Solution
In the first step, 2-methylbutane nitrile is protonated by HCl. In the next step, the nucleophilic attack of water on the protonated nitrile occurs and the accompanying proton transfer yields a protonated aminoketone. Another nucleophilic attack on the carbonyl carbon of the protonated aminoketone in the next step and the subsequent proton transfer yields a protonated diol intermediate which eliminates ammonia and a proton in the subsequent steps to yield 2-methylbutanoic acid.
The products of the reaction are ammonia and 2-methylbutanoic acid.
The mechanism of the reaction is given below.

d)
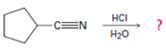
Interpretation:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step, is to be given.
Concept introduction:
The acid protonates the nitrogen of the nitrile group initially. The nucleophilic attack by water and subsequent proton transfer will yield an intermediate. Another nucleophilic attack by water on the intermediate and yet another proton transfer produces another intermediate. The intermediate loses ammonia to produce the protonated acid which deprotonates to yield the acid.
To give:
The products of the reaction, the acid-catalyzed nitrile hydrolysis, along with all the steps involved using curved arrows to represent electron flow in each step.
Answer to Problem 25MP
The products of the reaction are ammonia and cyclopentanecarboxylic acid.
The mechanism of the reaction is given below.
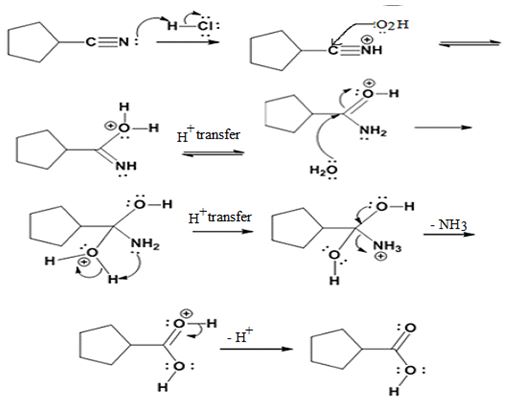
Explanation of Solution
In the first step, cyclopentanenitrile is protonated by HCl. In the next step, the nucleophilic attack of water on the protonated nitrile occurs and the accompanying proton transfer yields a protonated aminoketone. Another nucleophilic attack on the carbonyl carbon of the protonated ketone in the next step and the subsequent proton transfer yields a protonated aminodiol intermediate which eliminates ammonia and a proton in the subsequent steps to yield cyclopentanecarboxylic acid.
The products of the reaction are ammonia and cyclopentanecarboxylic acid.
The mechanism of the reaction is given below.

Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEM.(LL)-W/OWL V2 >CUSTOM<
- Enamines normally react with methyl iodide to give two products: one arising from alkylation at nitrogen and the second arising from alkylation at carbon. For example, Heating the mixture of C-alkylation and N-alkylation products gives only the product from C-alkylation. Propose a mechanism for this isomerization.arrow_forwardThe base-promoted rearrangement of an -haloketone to a carboxylic acid, known as the Favorskii rearrangement, is illustrated by the conversion of 2-chlorocyclohexanone to cyclopentanecarboxylic acid. It is proposed that NaOH first converts the a-haloketone to the substituted cyclopropanone shown in brackets and then to the sodium salt of cyclopentanecarboxylic acid. (a) Propose a mechanism for base-promoted conversion of 2-chlorocyclohexanone to the proposed intermediate. (b) Propose a mechanism for base-promoted conversion of the proposed intermediate to sodium cyclopentanecarboxylate.arrow_forwardNucleophilic aromatic substitution provides one of the common methods for making phenols. (Another method is discussedin Section 19-17.) Show how you would synthesize the following phenols, using benzene or toluene as your aromatic startingmaterial, and explain why mixtures of products would be obtained in some cases.(a) p-nitrophenol (b) 2,4,6-tribromophenol (c) p-chlorophenol(d) m-cresol (e) p-n-butylphenolarrow_forward
- On synthesis of esters via nucleophilic acyl substitution: How is excess alcohol eliminated from the crude product? Write the chemical equation involved in the reaction between the excess acid and NaHCO3. Given this, briefly explain why NaHCO3 is preferred over NaOH for the neutralization of excess acid.arrow_forwardWrite the equilibrium-constant expressions and obtainnumerical values for each constant in. (a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of hypochlorous acid,HClO. (c) the acidic dissociation of methyl ammoniumhydrochloride, CH3NH3Cl. (d) the basic dissociation of NaNO2. (e) the dissociation of H3AsO3to H3O+and AsO33-. (f) the reaction of C2O42-with H2O to give H2C2O4and OH-. show solutionarrow_forwardWrite the formula of reagents used in the following reactions:(i) Bromination of phenol to 2,4,6-tribromophenol(ii) Hydroboration of propene and then oxidation to propanol.(b) Arrange the following compound groups in the increasing order of their property indicated:(i) p-nitrophenol, ethanol, phenol (acidic character)(ii) Propanol, Propane, Propanal (boiling point)arrow_forward
- Can you please check my work on the following ochem reaction scheme and let me know if it is correct or what is wrong... the question was: Consider 3,4-dimethylpiperidine being subjected to the following: Step 1: CH3I (excess); Step 2: NaOH, heat Step 3: CH3I (excess); Step 4: NaOH, heat Provide the bond line structures for the major organic product obtained in each step and discuss the regiochemistry for Step 2.arrow_forwardWrite the structure of the major organic product isolated from the reaction of 3-hexyne with (a) Hydrogen (2 mol), platinum (b) Hydrogen (1 mol), Lindlar palladium (c) Hydrogen chloride (1 mol) (d) Hydrogen chloride (2 mol) (e) Chlorine (1 mol) (f) Chlorine (2 mol) (g) Aqueous sulfuric acid, mercury(II) sulfate (h) Ozone followed by hydrolysisarrow_forward(i) Name, draw and describe the organic product of the reaction between 2-methylbut-1-ene and H2O in the presence of H2SO4 and provide a clear rationale as to why this is the major product of the reaction. (ii) The elimination reaction between 2-bromobutane and NaOCH2CH3 gives two organic products. Draw a mechanism for the reaction which produces the major organic elimination product and provide a rationale as to why that is the major product.arrow_forward
- Given the chemical equation in the synthesis of benzoic acid from toluene and potassium permanganate: C7H8(I) + 2KMnO4(aq) → KC7H5O2 + 2MnO2 (s) + KOH (aq) + H2O (I) KC2H5O2 (aq) + HCl (aq) → C7H8O2 (s) + KCl (aq) MW: C7H8 = 92.14 KMnO4 = 158.03 MnO2 = 86.94 KC7H5O2 = 160.21 C7H8O2 = 122 Density: C7H8 = 0.87 g/mL KMnO4 = 2.7 g/mL KC7H5O2 = 1.5 g/mL C7H8O2 = 1.27 g/mL If 1 mL of toluene and 4 mL of 50% w/v potassium permanganate solution are used to synthesize the benzoic acid in a reflux set-up, calculate the theoretical yield of benzoic acid. Show your complete solution and underline your final answer.arrow_forwardWhich of the following statements is correct? A) The haloform reaction proceeds under very difficult conditions and the yield is very low. B) The haloform reaction is used only for the identification of compounds containing secondary alcohol groups. C) Methyl ketones or alcohols are oxidized with halogens in acidic solutions to give carboxylic acids and the appropriate haloform product. D) Enolate anions react rapidly with halogens to give alpha-halocarbonyl compounds.arrow_forwardFor the base-catalysed hydrolysis of 3-bromo-3-methylhexane (i.e. reaction with the nucleophile OH-): For the reaction intermediate, draw its structure and give the VSEPR description of the geometry at the reaction centre Give the names and draw the structures of the two reaction products. Explain your conclusions.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

