
(a)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
The ester is hydrolyzed to the
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
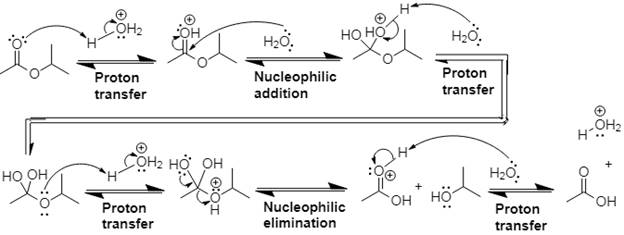
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed ester hydrolysis.
(b)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid-catalyzed transesterification, and a new ester is formed.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
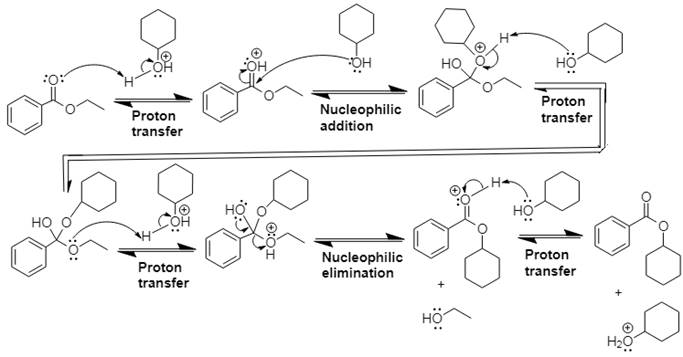
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2,
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed transesterification.
(c)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed amide hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
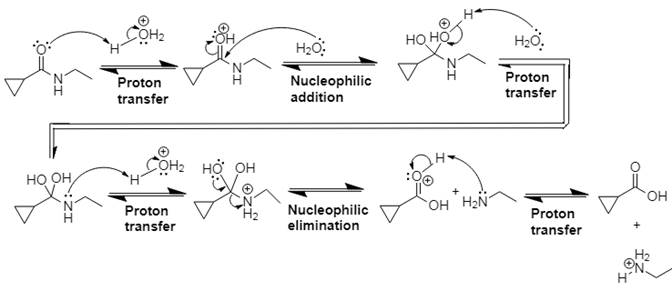
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed amide hydrolysis.
(d)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed ester hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of alcohol. Proton transfer steps are incorporated to avoid the appearance of strong bases.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
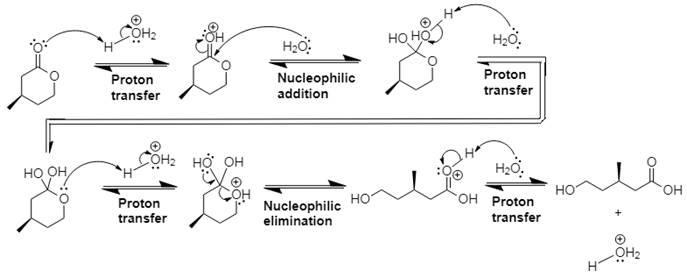
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed ester hydrolysis.
(e)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is Fischer esterification reaction.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
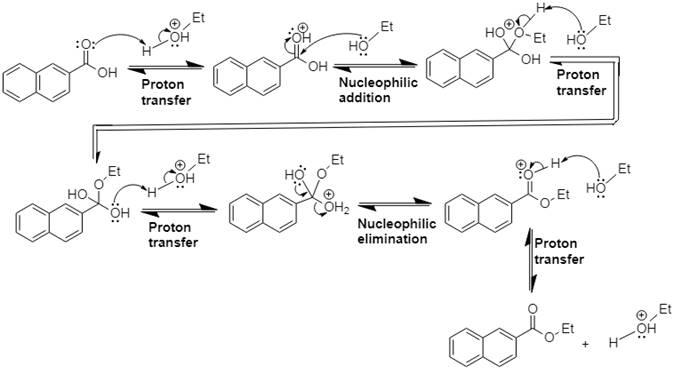
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the acid’s carbonyl group, and in Step 2,
The complete, detailed mechanism and the overall product for the given reaction is drawn by Fischer esterification reaction.
(f)
Interpretation:
The complete, detailed mechanism and the overall product for the given reaction is to be drawn.
Concept introduction:
This is an acid catalyzed amide hydrolysis which produces a carboxylic acid. Water acts as the nucleophile in this acid-catalyzed nucleophilic addition–elimination mechanism, and the leaving group is a molecule of amine. Proton transfer steps are incorporated to avoid the appearance of strong bases.
Answer to Problem 21.14P
The mechanism and product for the given reaction is:
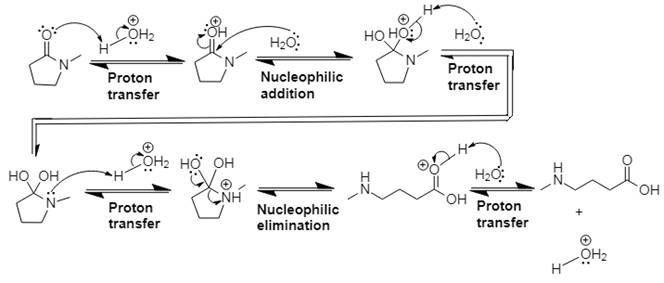
Explanation of Solution
In Step 1 of the mechanism, the acid protonates the ester’s carbonyl group, and in Step 2, water attacks the carbonyl
The complete, detailed mechanism and the overall product for the given reaction is drawn by acid-catalyzed amide hydrolysis.
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry: Principles And Mechanisms: Study Guide/solutions Manual (second)
- Draw the complete, detailed mechanism for the reaction shown here. Will the product be optically active? Explain.arrow_forwardOH Draw the complete, detailed mechanism for the reaction shown here. (See Problem 17.63.) NaBH4 Ethanolarrow_forwardDraw the complete, detailed mechanism for the following reaction along with the major product. Cl2 HOẠCarrow_forward
- Draw the complete, detailed mechanism for the following reaction.arrow_forwarda) Draw a complete, detailed mechanism for the following reaction and label the rate- determining step. H,SO, b) Show how the following molecule can be synthesized from benzene ZEU-arrow_forwardDraw a complete, detailed mechanism for the reaction shown here.arrow_forward
- Draw the complete, detailed mechanism for the reaction shown here, and explain why nucleophilic attack takes place predominantly at the epoxide carbon that is attached to the vinyl group. HCI H,0 ОН 63%arrow_forwardOne way to synthesize diethyl ether is to heat ethanol in the presence of a strong acid, as shown here. Draw a complete, detailed mechanism for this reaction.arrow_forwardDraw the complete, detailed mechanism for the reaction shown here and draw the major organic product. NH3 C,H15N H2O, Aarrow_forward
- Draw the complete, detailed mechanism for the following reaction.arrow_forwardDraw the detailed mechanism and the product for the following reaction. Be sure to include stereochemistry, if applicable. Brarrow_forwardDraw all the detailed plausible mechanism and all relevant resonance structures of the reaction below. Make sure to explain the regiochemistry of the product.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
