
(a)
Interpretation:
The major product of the reaction between propionyl chloride and
Concept introduction:
Acid chloride is a acid of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride and
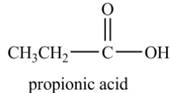
Explanation of Solution
Propionyl chloride undergoes hydrolysis reaction to from propionic acid. The water molecule acts as a nucleophile and attacks on the carbonyl carbon atom of propionyl chloride. The resultant compound lose
The corresponding
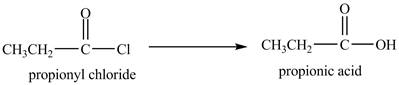
Figure 1
The major product of the reaction between propionyl chloride and
(b)
Interpretation:
The major product of the reaction between propionyl chloride, ethanethiol, and pyridine, at
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride, ethanethiol, and pyridine, at
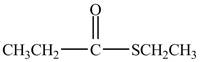
Explanation of Solution
Propionyl chloride is a acyl chloride. It has a electrophilic carbonyl carbonyl carbon atom. The ethanethiol molecule acts as a nucleophile and attacks on the carbonyl carbon atom of propionyl chloride. The resultant compound lose
The corresponding chemical reaction is shown below.

Figure 2
The major product of the reaction between propionyl chloride, ethanethiol, and pyridine, at
(c)
Interpretation:
The major product of the reaction between propionyl chloride,
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride,
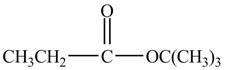
Explanation of Solution
Propionyl chloride is an acyl chloride. It has an electrophilic carbonyl carbon atom. The compound
The corresponding chemical reaction is shown below.

Figure 3
The major product of the reaction between propionyl chloride,
(d)
Interpretation:
The major product of the reaction between propionyl chloride and
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride and
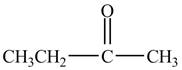
Explanation of Solution
Propionyl chloride is an acyl chloride. It has an electrophilic carbonyl carbon atom. The compound
The corresponding chemical reaction is shown below.
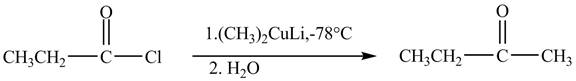
Figure 4
The major product of the reaction between propionyl chloride and
(e)
Interpretation:
The major product of the reaction between propionyl chloride and ![]() sulfur poison) is to be predicated.
sulfur poison) is to be predicated.
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride and ![]() sulfur poison) is shown below.
sulfur poison) is shown below.
Explanation of Solution
Propionyl chloride is an acyl chloride. It has an electrophilic carbonyl carbon atom. propionyl chloride reduces similary as carbonylic acid with ![]() sulfur poison to an
sulfur poison to an
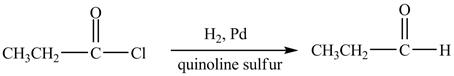
Figure 5
The major product of the reaction between propionyl chloride and ![]() sulfur poison) is shown in Figure 5
sulfur poison) is shown in Figure 5
(f)
Interpretation:
The major product of the reaction between propionyl chloride and
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride and
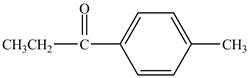
Explanation of Solution
Propionyl chloride is an acyl chloride. It has an electrophilic carbonyl carbon atom. The compound undergoes Fredal craft acylation reaction with toluene in the presence of catalyst
The corresponding chemical reaction is shown below.
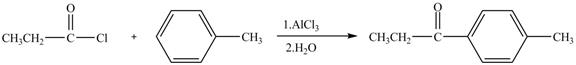
Figure 6
The major product of the reaction between propionyl chloride and
(g)
Interpretation:
The major product of the reaction between propionyl chloride and
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride and
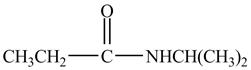
Explanation of Solution
Propionyl chloride is an acyl chloride. It has an electrophilic carbonyl carbon atom. The compound
The corresponding chemical reaction is shown below.
![]()
Figure 7
The major product of the reaction between propionyl chloride and
(h)
Interpretation:
The major product of the reaction between propionyl chloride and sodium benzoate is to be predicated.
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride and sodium benzoate is shown below.
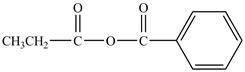
Explanation of Solution
Propionyl chloride is an acyl chloride. It has an electrophilic carbonyl carbon atom. Th The compound sodium benzoate reacts with propionyl chloride. The resultant compound lose
The corresponding chemical reaction is shown below.
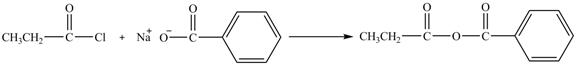
Figure 8
The major product of the reaction between propionyl chloride and sodium benzoate is shown in Figure 8.
(i)
Interpretation:
The major product of the reaction between propionylchloride,
Concept introduction:
Acid chloride is a type of carboxylic acid derivatives. The general formula of acid chloride is
Answer to Problem 21.33AP
The major product of the reaction between propionyl chloride,
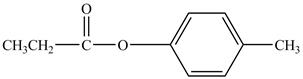
Explanation of Solution
Propionyl chloride is an acyl chloride. It has an electrophilic carbonyl carbon atom. Th The compound
The corresponding chemical reaction is shown below.

Figure 9
The major product of the reaction between propionyl chloride,
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Amines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forwardCompound H (C8H6O3) gives a precipitate when treated with hydroxylamine in aqueous ethanol and a silver mirror when treated with Tollens solution. Following is its 1H-NMR spectrum. Deduce the structure of compound H.arrow_forwardCompound X is optically inactive and has the formula C 16H 16Br 2. On treatment with strong base, X gives hydrocarbon Y, C 16H 14. Compound Y absorbs 2 equivalents of hydrogen when reduced over a palladium catalyst and reacts with ozone to give two fragments. One fragment Z, is an aldehyde with formula C 7H 6O. The other fragment is glyoxal, (CHO)2. Which of the following answers is correct? Select all that are correct.arrow_forward
- Give the expected organic product when phenylacetic acid, PhCH2COOH, is treated with reagent Q.)LiAlH4 followed by H2Oarrow_forward2-bromo-2-methylbutane undergoes hydrolysis reaction with water, H2O toform compound W. Compound X and compound Y are produced when 2-bromo-2-methylbutane undergoes elimination reaction with alcoholic ofsodium hydroxide, NaOH. (i) Draw the structural formula of compounds W, X and Yarrow_forwardThe compound acetophenone has a very similar molar mass to that of benzoic acid and benzamide. However, acetophenone has a much lower m.p. (20 °C) than both such that, by contrast, it is a liquid at room temperature. By considering intermolecular forces and comparing functional group structure, account for this big difference in physical properties.arrow_forward
- A synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardThree isomeric pentanols with unbranched carbon chains exist. Which of these isomers, upon dehydration at 180C, yields only 1-pentene as a product?arrow_forwardCompound 1 is an anticoagulant that is extracted from, among other plants, the sweetclover plant, Melilotus Officinalis. However, the compound is moderately toxic and alsofinds use as a rodenticide. Name this compoundarrow_forward
- (a) Arrange the following compounds in an increasing order of their indicated property :(i) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)(ii) CH3CH2CH (Br) COOH, CH3CH (Br) CH2COOH,(CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)(b) How would you bring about the following conversions :(i) Propanone to Propene (ii) Benzoic acid to Benzaldehyde(iii) Bromobenzene to 1-phenylethanolarrow_forward(a) Draw the structures of the following compounds :(i) 4-Chloropentan-2-one (ii) p-Nitropropiophenone(b) Give tests to distinguish between the following pairs of compounds :(i) Ethanal and Propanal (ii) Phenol and Benzoic acid(iii) Benzaldehyde and Acetophenonearrow_forwardThe formula for the alarm pheromone for one species of ant is C7 H7 O. When treated with I2 and NaOH, this pheromone yields iodoform and n-hexanoic acid. What is the structure of the pheromone?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,



