
Concept explainers
(a)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Sodiumborohydride is a metal reducing agent. It reduces
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
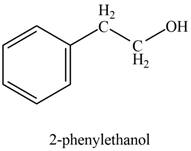
Explanation of Solution
In the presence of sodium borohydride and methanol, phenylacetaldehyde gets reduced to
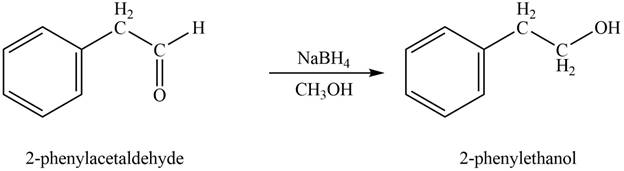
Figure 1
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 1.
(b)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Lithium aluminum hydride is a strong reducing agent. Treatment of carbonyl compounds with lithium aluminum hydride followed by water yields alcohols. It reduces aldehyde into primary alcohol.
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
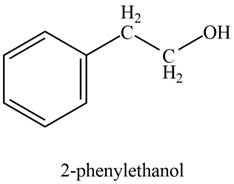
Explanation of Solution
In the presence of lithium aluminium hydride followed by hydrolysis, phenylacetaldehyde gets reduced to
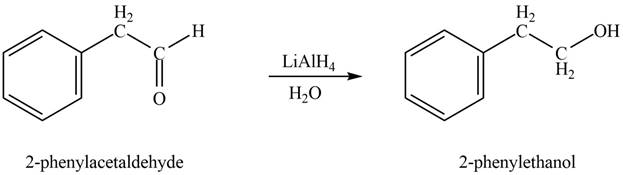
Figure 2
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 2.
(c)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Grignard reagents are
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
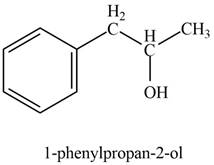
Explanation of Solution
In the presence of Grignard reagent followed by hydrolysis, phenylacetaldehyde forms primary alcohol. The corresponding chemical reaction is shown below.
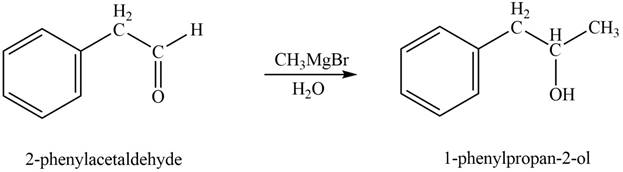
Figure 3
The product formed is
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 3.
(d)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Nucleophilic addition reaction is a type of organic reaction in which the nucleophile is added to an electrophilic site. In case of carbonyl compounds, the carbonyl carbon acts as an electrophilic site.
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
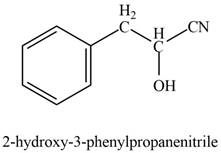
Explanation of Solution
Phenylacetaldehyde reacts with sodium cyanide followed by protonation to form
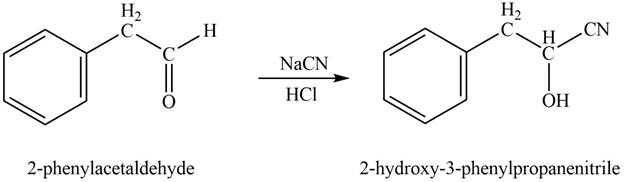
Figure 4
(e)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
In Wittig reaction, aldehyde can be directly converted to
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
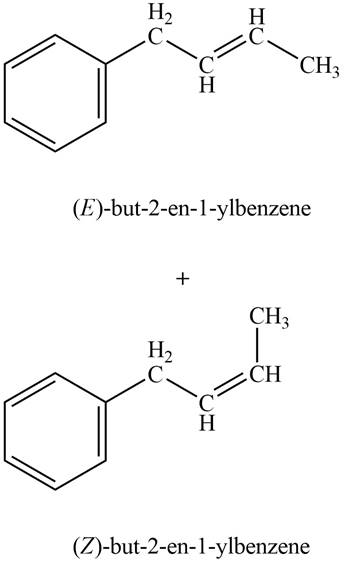
Explanation of Solution
Phenylacetaldehyde on reaction with Wittig reagent
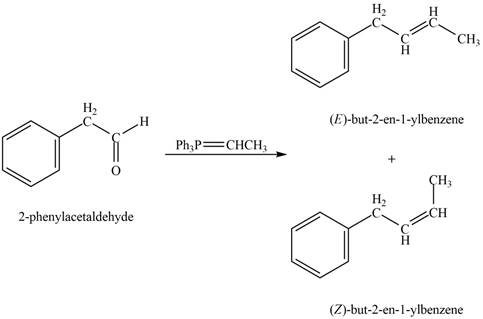
Figure 5
The products formed are
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 5.
(f)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Aldehyde or ketone on reaction with primary
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
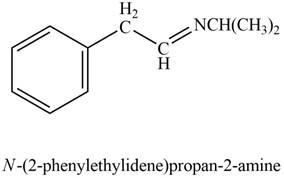
Explanation of Solution
Phenylacetaldehyde reacts with
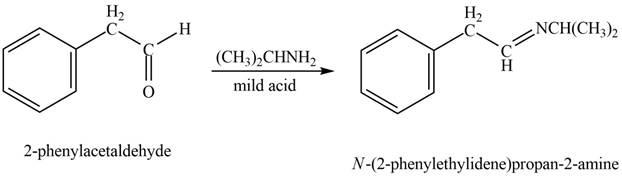
Figure 6
The product formed is
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 6.
(g)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Aldehyde or ketone on reaction with secondary amine forms enamine. The nucleophilic attack of secondary amine on carbonyl carbon results in the formation of intermediate carbinolamine. This intermediate loses water to form an enamine. In this case, the elimination occurs across two adjacent carbon atoms to form new carbon-carbon double bond.
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
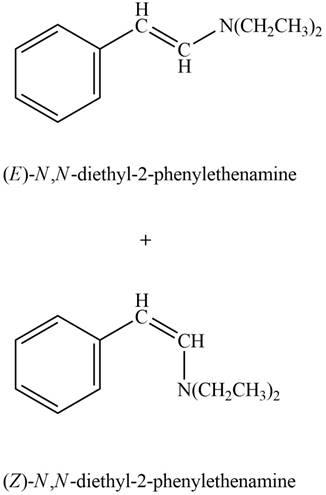
Explanation of Solution
Phenylacetaldehyde reacts with
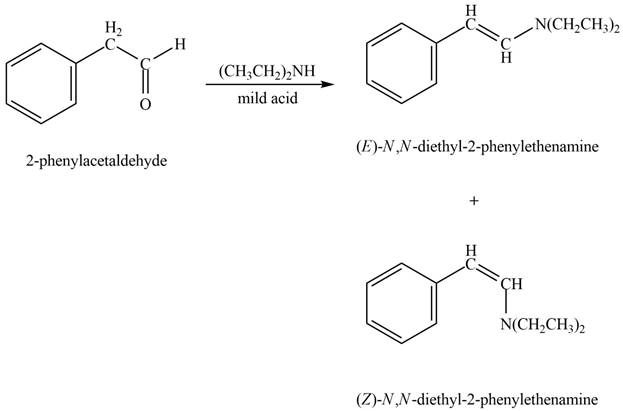
Figure 7
The products formed are
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 7.
(h)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions take place in presence of acids.
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
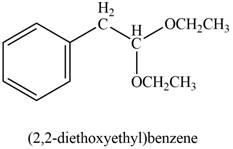
Explanation of Solution
Phenylacetaldehyde reacts with excess of ethanol in presence of acid to form acetal. The corresponding reaction is as follows,
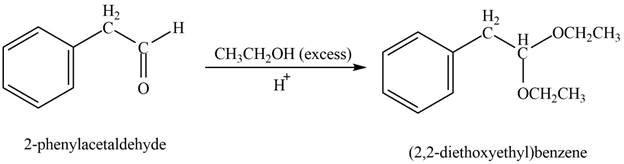
Figure 8
The product formed is (
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 8.
(i)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Aldehyde or ketone on reaction with secondary amine forms enamine. The nucleophilic attack of secondary amine on carbonyl carbon results in the formation of intermediate carbinolamine. This intermediate loses water to form an enamine. In this case the elimination occurs across two adjacent carbon atoms to form new carbon-carbon double bond.
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
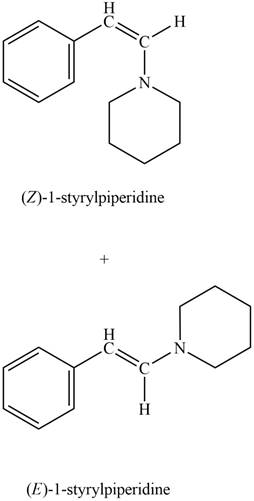
Explanation of Solution
Phenylacetaldehyde reacts with piperidine in presence of mild acid to form E and Z isomers of
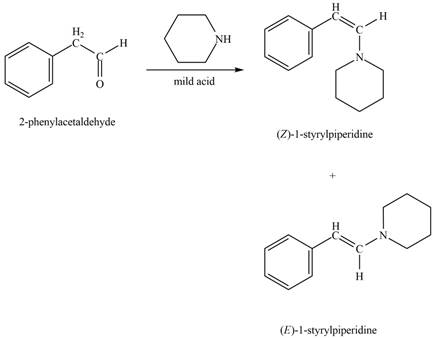
Figure 9
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 9.
(j)
Interpretation:
The product formed on treatment of phenylacetaldehyde with given reagent is to be predicted.
Concept introduction:
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions take place in presence of acids.
Answer to Problem 21.45P
The product formed on treatment of phenylacetaldehyde with given reagent is,
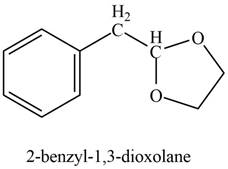
Explanation of Solution
Phenylacetaldehyde reacts with ethane-
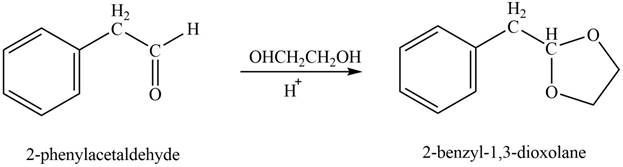
Figure 10
The product formed is
The product formed on treatment of phenylacetaldehyde with given reagent is shown in figure 10.
Want to see more full solutions like this?
Chapter 21 Solutions
Connect Access Card For Organic Chemistry
- Draw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent (a, b and c)arrow_forwardDraw the products formed when p-methylaniline (p-CH3C6H4NH2) is treated with each reagent. a. HCl b. CH3COCl c. (CH3CO)2O d. excess CH3I e. (CH3)2C = O f. CH3COCl, AlCl3 g. CH3CO2H h. NaNO2, HCl i. Part (b), then CH3COCl, AlCl j. CH3CHO, NaBH3CNarrow_forward
- Draw the products formed when phenol (C6H5OH) is treated with each set of reagents. a. [1] HNO3, H2SO4; [2] Sn, HCl b. [1] (CH3CH2)2CHCOCl, AlCl3; [2] Zn(Hg), HCl c. [1] CH3CH2Cl, AlCl3; [2] Br2, hν d. [1] (CH3)2CHCl, AlCl3; [2] KMnO4arrow_forwardEleostearic acid, C18H30O2, is a rare fatty acid found in the tung oil used for finishing furniture. On ozonolysis followed by treatment with zinc, eleostearic acid furnishes one part pentanal, two parts glyoxal (OHC-CHO), and one part 9-oxononanoic acid [OHC(CH2)7CO2H]. What is the structure of eleostearic acid?arrow_forwardwhich reagents complete the reaction?arrow_forward
- Draw the products formed when phenol (C6H5OH) is treated with each set of reagents.a. [1] HNO3, H2SO4; [2] Sn, HCIb. [1] (CH3CH2)2CHCOCI, AlCl3; [2] Zn(Hg), HCIc. [1] CH3CH2CI, AlCl3; [2] Br2, hvd. [1] (CH3)2CHCI, AlCl3; [2] KMnO4arrow_forwardDraw the products formed when phenol (C6H5OH) is treated with following set of reagents. [1] CH3CH2Cl, AlCl3; [2] Br2, hνarrow_forwardA. OsO4 and NMO B. Br2 and H20 C. Hg(OAc)2, H2O and NaBH4, NaOH D. RCO3H E. BH3-THF and H2O2, NaOH Which reagent will complete this reaction?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

