
What reagents are needed to convert each compound to
a.![]() b.
b.![]() c.
c.
(a)
Interpretation: The reagents needed to convert given compound to
Concept introduction: Potassium dichromate is a strong oxidizing agent. Potassium dichromate is used in many organic reactions to oxidize organic compounds. Chromium atom present in Potassium chromate itself gets reduced from oxidation state six to a lower oxidation state and oxidizes the other compound.
Answer to Problem 21.60P
The reagent needed to convert given compound to
Explanation of Solution
The given compound can be converted into
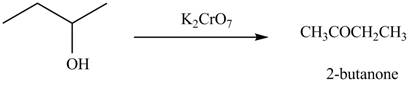
Figure 1
The reagent needed to convert given compound to
(b)
Interpretation: The reagents needed to convert given compound to
Concept introduction: Gilman reagent is an organometallic compound. Gilman reagent is lithium dialkylcuprate that is used to add alkyl group in an organic compound. Gilman reagent reacts with any organic halide and substitute the halide group by an alkyl group.
Answer to Problem 21.60P
The reagents needed to convert given compound to
Explanation of Solution
The given compound can be converted into
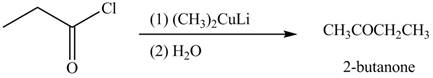
Figure 2
The reagents needed to convert given compound to
(c)
Interpretation: The reagents needed to convert given compound to
Concept introduction: Gilman reagent is an organometallic compound. Gilman reagent is lithium dialkylcuprate that is used to add alkyl group in an organic compound. Gilman regent reacts with any organic halide and substitute the halide group by alkyl group.
Answer to Problem 21.60P
The reagent needed to convert given compound to
Explanation of Solution
The given compound can be converted into
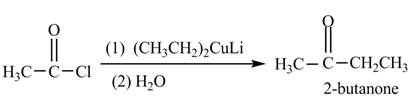
Figure 3
The reagent needed to convert given compound to
(d)
Interpretation: The reagents needed to convert given compound to
Concept introduction: When an alkyne reacts with mercury sulfate solution in a strong acidic medium a ketone is formed and this reaction is known as the oxymercuration of alkynes. The oxymercuration of alkyne is a very effective method to convert an alkyne into a ketone.
Answer to Problem 21.60P
The reagent needed to convert given compound to
Explanation of Solution
The given compound can be converted into
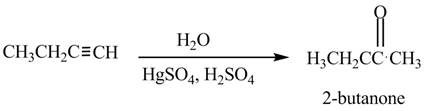
Figure 4
The reagent needed to convert given compound to
(e)
Concept introduction: When an alkyne reacts with mercury sulfate solution in a strong acidic medium a ketone is formed and this reaction is known as the oxymercuration of alkynes. The oxymercuration of alkyne is a very effective method to convert an alkyne into a ketone.
Answer to Problem 21.60P
The reagent needed to convert given compound to
Explanation of Solution
The given compound can be converted into
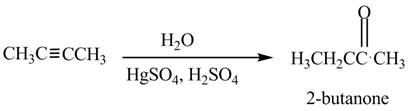
Figure 5
The reagent needed to convert given compound to
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry -Study Guide / Solution Manual (Custom)
- Isooctane is the common name of the isomer of C8H18 used as the standard of 100 for the gasoline octane rating: (a) What is the IUPAC name for the compound? (b) Name the other isomers that contain a five-carbon chain with three methyl substituents.arrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forwardWhat is the name of the alkene? CH3CH=CHCH=CH2arrow_forward
- ALCOHOLS 1. WHY IS ETHANOL MORE SOLUBLE IN WATER THAN 1-HEXANOL? 2. WHAT IS DENATURED ALCOHOL? AND WHY IS ALCOHOL DENATURED? ETHER 1. WHY DOES DIETHYL ETHER HAVE MUCH LOWER BOILING POINT THAN 1-BUTANOL?arrow_forwardIs this correct? Benzene reacts with CH3CH(CH2CH2CH3)CH(Cl)CH2CH3, catalyst ALCl3arrow_forwardHCl (aq), Zn(Hg) Br2, FeBr3 NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heat CH3Cl, AlCl3 Dilute H3O+ ClCO(CH2)2CH3, AlCl3 NaCCCH2CH3 HNO3, H2SO4 2-butanonearrow_forward
- Draw the product of benzene and Cl2/FeCl3arrow_forward1. Draw the structure for each compound. a.(3R)-3-methylhexane b. (3R,5S,6R)-5-ethyl-3,6-dimethylnonanearrow_forwardn-Butyl methyl ether is an isomer of MTBE and has a boiling point of 70 oC. Explain why the boiling point is significantly different compared to MTBE.arrow_forward
- 6. Draw the correct structures for the following: a. what is the correct structure of 2-heptene? b. what is the correct structure of 4-octanone? c. what is the correct structure of dipropyl ether? d. what is the correct structure of 3-ethyl hexane? e. what is the correct structure of 2,2 dimethyl 1-hexanol?arrow_forwardWhich alkyl halide has the highest boiling point? A. CH3BrB. CH3FC. CH3ClD. CH3larrow_forwardWhat reagents are needed to convert 1-ethylcyclohexene into (a) 1-bromo-2-ethylcyclohexane; (b) 1-bromo-1-ethylcyclohexane; (c) 1,2-dibromo-1-ethylcyclohexane?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





