
(a)
Interpretation:
The type of decay and the isotope which undergoes that particular decay should be identified.
Concept Introduction:
Nuclear reaction is a physical process in which there is a change in identity of an atomic nucleus. Natural radioactive decays, artificial radioactive decays... are considered as nuclear reactions because these processes make changes in the identity of an atomic nucleus.
Common particles in radioactive decay and nuclear transformations are mentioned below,
There are various types of nuclear processes. The changes in
An isotope can be represented using
(a)
Explanation of Solution
Given diagram for the decay is,
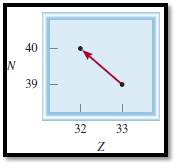
Figure 1
From the diagram the isotope can be identified,
Atomic number is decreased from
The nuclear reaction can be represented as,
(b)
Interpretation:
The type of decay and the isotope which undergoes that particular decay should be identified.
Concept Introduction:
Nuclear reaction is a physical process in which there is a change in identity of an atomic nucleus. Natural radioactive decays, artificial radioactive decays... are considered as nuclear reactions because these processes make changes in the identity of an atomic nucleus.
Common particles in radioactive decay and nuclear transformations are mentioned below,
There are various types of nuclear processes. The changes in atomic number and mass number accompanying radioactive decay are mentioned below,
(b)
Explanation of Solution
Given diagram for the decay is,
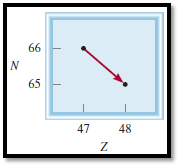
Figure 2
From the diagram the isotope can be identified,
Atomic number is increased from
The nuclear reaction can be represented as,
(c)
Interpretation:
The type of decay and the isotope which undergoes that particular decay should be identified.
Concept Introduction:
Nuclear reaction is a physical process in which there is a change in identity of an atomic nucleus. Natural radioactive decays, artificial radioactive decays... are considered as nuclear reactions because these processes make changes in the identity of an atomic nucleus.
Common particles in radioactive decay and nuclear transformations are mentioned below,
There are various types of nuclear processes. The changes in atomic number and mass number accompanying radioactive decay are mentioned below,
(c)
Explanation of Solution
Given diagram for the decay is,
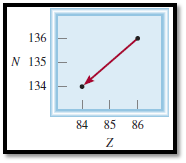
Figure 3
From the diagram the isotope can be identified.
Atomic number is decreased from
The nuclear reaction can be represented as,
Want to see more full solutions like this?
Chapter 21 Solutions
Loose Leaf Version for Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





