
Concept explainers
(a)
Interpretation:
Electronic configuration of the following metals has to be written –
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
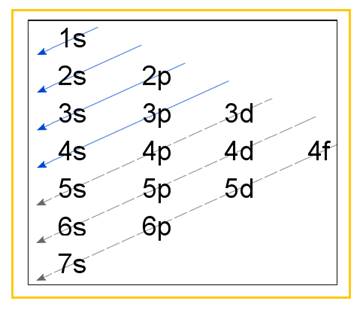
Figure 1
The terms
(a)
Answer to Problem 22E
Electronic configuration of
Explanation of Solution
The above electronic configuration corresponds to that of Argon till
(b)
Interpretation:
Electronic configuration of the following metals has to be written –
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –

Figure 1
The terms
(b)
Answer to Problem 22E
Electronic configuration of
Explanation of Solution
Atomic number of Cadmium is
The above electronic configuration corresponds to that of Argon till
(c)
Interpretation:
Electronic configuration of the following metals has to be written –
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
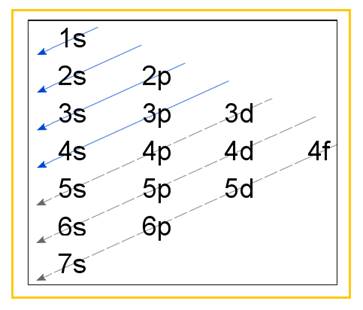
Figure 1
The terms
(c)
Answer to Problem 22E
Electronic configuration of
Explanation of Solution
Atomic number of Zirconium is
The electronic configuration of
The above electronic configurations correspond to that of Argon till
(d)
Interpretation:
Electronic configuration of the following metals has to be written –
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
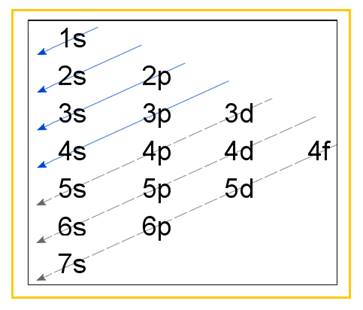
Figure 1
The terms
(d)
Answer to Problem 22E
Electronic configuration of
Explanation of Solution
Atomic number of Osmium is
Os2+ and Os3+ are formed when Osmium loses two electrons and three electrons respectively. Accordingly the electronic configuration of Os2+ is –
The above electronic configuration corresponds to that of Xenon till
The electronic configuration of
The above electronic configuration corresponds to that of Xenon till
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry: Cengage Technology Edition
- The complex ion PdCl42is diamagnetic. Propose a structure for PdCl42.arrow_forwardThe transition metals form a class of compounds called metal carbonyls, an example of which is the tetrahedral complex Ni(CO)4. Given the following thermodynamic data (at 298 K): (a) Calculate the equilibrium constant for the formation of Ni(CO)4(g) from nickel metal and CO gas. (b) Is the reaction of Ni(s) and CO(g) product- or reactant-favored at equilibrium? (c) Is the reaction more or less product-favored at higher temperatures? How could this reaction be used in the purification of nickel metal?arrow_forwardThe standard reduction potential for the reaction [Co( H 2 O)6]3+(aq)+e[CO( H 2 O)6]2+(aq) is about 1.8 V. The reduction potential for the reaction [Co( NH 3 )6]3+(aq)+e[Co( NH 3 )6]2+(aq) is +0.1 V. Calculate the cell potentials to show whether the complex ions,. [Co( H 2 O)6]2+ and or [Co( NH 3 )6]2+, can be oxidized to the corresponding Cobalt (III) complex by oxygen.arrow_forward
- a. In the absorption spectrum of the complex ion Cr(NCS)63, there is a band corresponding to the absorption of a photon of light with an energy of 1.75 104 cm-1. Given 1 cm1 = 1.986 1023 J, what is the wavelength of this photon? b. The CrNC bond angle in Cr(NCS)63 is predicted to be 180. What is the hybridization of the N atom in the Ncs- ligand when a Lewis acid-base reaction occurs between Cr3+ and NCs- that would give a 180 CrNC bond angle? Cr(NCS)63 undergoes substitution by ethylenediamine (en) according to the equation Cr(NCS)63+2enCr(NCS)2(en)2++4NCS Does Cr(NCS)2(en)2+ exhibit geometric isomerism? Does Cr(NCS)2(en)2+ exhibit optical isomerism?arrow_forwardWhy do ligands cause the d orbitals of a transition metal ion to cease being degenerate? A. New molecular orbitals are formed via atomic orbital overlapt B. There is repulsion between the ligands and the d orbitals. C. They destabilize the ion nucleus D. Donation of electrons to the metal ion results in redox chemistryarrow_forwardWhat is a Chelate ligand? Give one example.arrow_forward
- (a) Calculate the number of unpaired electrons in the following gaseous state ions: Mn2+, Cr3+, V3+ and Fe2+ which one of these in the most stable in aqueous solutions? (At. nos. V = 23, Cr = 24, Mn = 25, Fe = 26) (b) Explain the following observations: (i) The transition metal ions are usually coloured in aqueous solutions. (ii) Cu(I) is not stable in an aqueous solution. (iii) The highest oxidation state of a transition metal is exhibited in its oxide or fluoride.arrow_forwardWhich has a larger LFSE between this pair, why? [Fe(CN)6]3– or [Ru(CN)6]3–? Please explain.arrow_forwardHow would you account for the following?(i) Many of the transition elements are known to form interstitial compounds.(ii) The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group members of the second (4d) series.(iii) Lanthanoids form primarily +3 ions, while the actinoids usually have higher oxidation states in their compounds, +4 or even +6 being typical.arrow_forward
- (a) Why do transition elements show variable oxidation states? (i) Name the element showing maximum number of oxidation states among the first series of transition metals from Sc (Z = 21) to Zn (Z = 30). (ii) Name the element which shows only +3 oxidation state. (b) What is lanthanoid contraction? Name an important alloy which contains some of the lanthanoid metals.arrow_forwardExplain the fundamentals of CFT applied to complexes. Indicate some applications, also mention some advantages and disadvantages of the theory.arrow_forwarda) what ions are present in the solution of K(s) and H2O(l)? b) what is the net ionic equation for K(s) and H2O(l)? c) would cesium metal be predicted to be more reactive or less reactive than sodium and potassium?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





