
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 82GQ
The
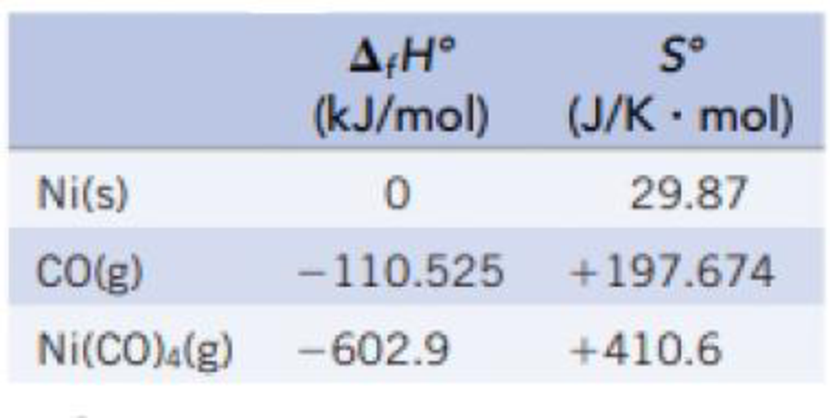
(a) Calculate the equilibrium constant for the formation of Ni(CO)4(g) from nickel metal and CO gas.
(b) Is the reaction of Ni(s) and CO(g) product- or reactant-favored at equilibrium?
(c) Is the reaction more or less product-favored at higher temperatures? How could this reaction be used in the purification of nickel metal?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The coordination complex [Cr(CO)6] forms colorless, diamagnetic crystals that melt at 90 °C. Given that [Cr(CO)6] is colorless, would you expect CO to be a weak-field or strong-field ligand?
Some metal complexes have a coordination number of 5. One such complex is Fe(CO)5, which adopts a trigonal bipyramidal geometry. What is the oxidation state of Fe in this compound?
When cobalt(II) chloride is treated, under certain condition, with the bidentate ligand, NH2CH2CH2NH2, (which can be represented by the symbol “en”), the compound [CoC12(en)2]Cl is formed.
a. What is the oxidation state of cobalt in the compound formed?
b. What is meant by the term ‘bidentate’ as applied to a ligand?
Chapter 22 Solutions
Chemistry & Chemical Reactivity
Ch. 22.4 - (a) What is the formula of a complex ion composed...Ch. 22.4 - (a) Determine the metals oxidation number and...Ch. 22.4 - Name the following coordination compounds. (a)...Ch. 22.5 - What types of isomers are possible for the...Ch. 22.6 - Prob. 22.5CYUCh. 22.7 - Prob. 22.6CYUCh. 22.7 - Prob. 1.1ACPCh. 22.7 - Copper has a face-centered cubic unit cell. If...Ch. 22.7 - Prob. 1.3ACPCh. 22.7 - If a patient is given 10.0 mg of cisplatin, what...
Ch. 22.7 - Prob. 2.2ACPCh. 22.7 - How are the d electrons of Pt distributed in a...Ch. 22.7 - What are the electron configurations for Nd and...Ch. 22.7 - Prob. 3.2ACPCh. 22.7 - Prob. 3.3ACPCh. 22.7 - Prob. 3.4ACPCh. 22 - Identify, based on the position in the periodic...Ch. 22 - Prob. 2PSCh. 22 - Prob. 3PSCh. 22 - Prob. 4PSCh. 22 - Prob. 5PSCh. 22 - Iron is the most abundant transition element in...Ch. 22 - Prob. 7PSCh. 22 - Prob. 8PSCh. 22 - Prob. 9PSCh. 22 - Prob. 10PSCh. 22 - Identify a cation of a first series transition...Ch. 22 - Match up the isoelectronic ions on the following...Ch. 22 - The lanthanide contraction is given as an...Ch. 22 - Prob. 14PSCh. 22 - Prob. 15PSCh. 22 - Prob. 16PSCh. 22 - Prob. 17PSCh. 22 - Prob. 18PSCh. 22 - Which of the following ligands is expected to be...Ch. 22 - One of the following nitrogen compounds or ions is...Ch. 22 - Prob. 21PSCh. 22 - Prob. 22PSCh. 22 - Prob. 23PSCh. 22 - Prob. 24PSCh. 22 - Prob. 25PSCh. 22 - Prob. 26PSCh. 22 - Prob. 27PSCh. 22 - Prob. 28PSCh. 22 - Prob. 29PSCh. 22 - Prob. 30PSCh. 22 - Give the name or formula for each ion or compound,...Ch. 22 - Prob. 32PSCh. 22 - Prob. 33PSCh. 22 - Prob. 34PSCh. 22 - Prob. 35PSCh. 22 - Prob. 36PSCh. 22 - Prob. 37PSCh. 22 - Prob. 38PSCh. 22 - Prob. 39PSCh. 22 - Prob. 40PSCh. 22 - Prob. 41PSCh. 22 - Prob. 42PSCh. 22 - Prob. 43PSCh. 22 - Prob. 44PSCh. 22 - Prob. 45PSCh. 22 - Prob. 46PSCh. 22 - Prob. 47PSCh. 22 - Prob. 48PSCh. 22 - Prob. 49PSCh. 22 - Prob. 50PSCh. 22 - In water, the titanium(III) ion, [Ti(H2O)6]3+, has...Ch. 22 - Prob. 52PSCh. 22 - Prob. 53GQCh. 22 - Prob. 54GQCh. 22 - How many unpaired electrons are expected for...Ch. 22 - Prob. 56GQCh. 22 - Which of the following complex ions is (are)...Ch. 22 - Prob. 58GQCh. 22 - How many geometric isomers are possible for the...Ch. 22 - For a tetrahedral complex of a metal in the first...Ch. 22 - Prob. 61GQCh. 22 - Prob. 62GQCh. 22 - Prob. 63GQCh. 22 - A platinum-containing compound, known as Magnuss...Ch. 22 - Prob. 65GQCh. 22 - Prob. 66GQCh. 22 - Prob. 67GQCh. 22 - How many geometric isomers of the complex ion...Ch. 22 - Prob. 69GQCh. 22 - Prob. 70GQCh. 22 - Prob. 71GQCh. 22 - The square-planar complex Pt(en)Cl2 has chloride...Ch. 22 - The complex [Mn(H2O)6]2+ has five unpaired...Ch. 22 - Experiments show that K4[Cr(CN)6] is paramagnetic...Ch. 22 - Give a systematic name or the formula for the...Ch. 22 - When CrCI3 dissolves in water, three different...Ch. 22 - Prob. 77GQCh. 22 - The glycinate ion, H2NCH2CO2, formed by...Ch. 22 - Prob. 79GQCh. 22 - Nickel and palladium both form complexes of the...Ch. 22 - The transition metals form a class of compounds...Ch. 22 - Cerium, as noted in Applying Chemical Principles:...Ch. 22 - Prob. 84GQCh. 22 - Two different coordination compounds containing...Ch. 22 - Prob. 89SCQCh. 22 - Prob. 90SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Four different octahedral chromium coordination compounds exist that all have the same oxidation state for chromium and have H2O and Cl as the ligands and counterions. When 1 mole of each of the four compounds is dissolved in water, how many moles of silver chloride will precipitate upon addition of excess AgNO3?arrow_forwardPlatinum(II) forms many complexes, among them those with the following ligands. Give the formula and charge of each complex. (a) two ammonia molecules and one oxalate ion (C2O42-) (b) two ammonia molecules, one thiocyanate ion (SCN-), and one bromide ion (c) one ethylenediamine molecule and two nitrite ionsarrow_forwardIdentify, based on the position in the periodic table, the actinide elements among those in the following list: Co, Cm, Cd, Ce, Cf.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY