
Concept explainers
Draw enol tautomer(s) for each compound. Ignore stereoisomers.
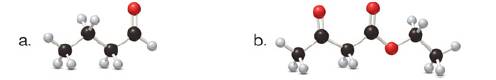
(a)
Interpretation: Enol tautomer(s) of the given compound is to be drawn.
Concept introduction: Tautomers are the isomers which differ only in the position of the hydrogens and electrons of electronegative element, generally oxygen. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
The very common example of tautomerism is Keto-enol tautomerism. It can be acid or base catalysed.
Answer to Problem 30P
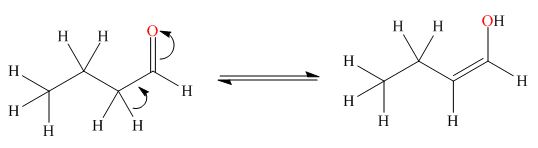
. The enol tautomer of this compound is shown below:
Explanation of Solution
Tautomers are isomers which differ only in the position of the protons and electrons of the compound. There is no change in the carbon skeleton of the compound. The ball and stick model as shown in Figure 1.
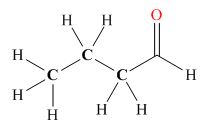
Figure 1
The enol tautomer of this compound is shown in Figure 2.

Figure 2
The tautomer of the given compound is showed in Figure 2.
(b)
Interpretation: Enol tautomer(s) of the given compound is to be drawn.
Concept introduction:
Tautomers are the isomers which differ only in the position of the hydrogens and electrons of electronegative element, generally oxygen. There is no change in the carbon skeleton of the compound. This phenomenon which involves simple proton transfer in an intramolecular fashion is known as tautomerism.
The very common example of tautomerism is Keto-enol tautomerism. It can be acid or base catalysed.
Answer to Problem 30P
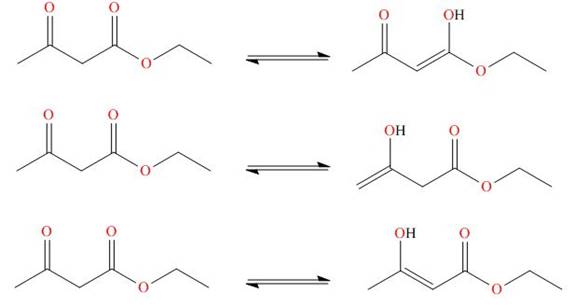
. The enol tautomer of this compound is shown below:
Explanation of Solution
Tautomers are isomers which differ only in the position of the protons and electrons of the compound. There is no change in the carbon skeleton of the compound. The ball and stick model as shown in Figure 3.
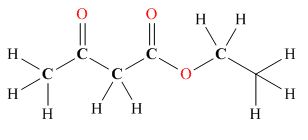
Figure 3
The enol tautomer of this compound is shown in Figure 4.

Figure 4
The tautomer of the given compound is showed in Figure 4.
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry (6th Edition)
- Draw all products formed by treatment of each dibromide (A and B) with one equivalent of NaNH2arrow_forwardDraw the structure of the acyclic polyhydroxy aldehyde that cyclizes to each hemiacetal.arrow_forwardDraw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs.a. NaBH4, CH3OHb. [1] LiAlH4; [2] H2Oc. [1] CH3MgBr (excess); [2] H2Od. [1] C6H5Li (excess); [2] H2Oe. Na2Cr2O7, H2SO4, H2Oarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
