
Concept explainers
Interpretation:
The use of periodic acid as a distinguishing reagent between an aldohexose and a ketohexose. Further, the product formed with each of the compounds and the respective number of molar equivalents of
Concept Introduction:
▸ The aldose or ketose carbon sugars that have six membered carbon atoms are referred to as aldohexoses and ketohexoses, respectively. Glucose is an example of aldohexose and fructose is an example of ketohexose. The structures of each have been described below:
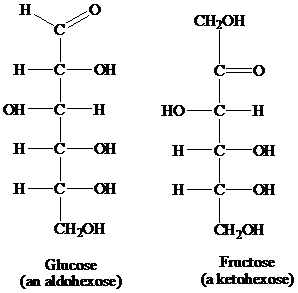
▸ Furthermore, both the aldohexoses and ketohexoses undergo periodate oxidation, in order to yield different products, as a result of the cleavage of the
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
- 21.80 Prednisolone is the synthetic glucocorticoid medicine most frequently prescribed to combat autoimmune diseases. Compare its structure to the natural glucocorticoid hormone cortisone. What are the similarities and differences in structure? НО. H₂C H₂C CH₂OH C=O -ОНarrow_forwardd-Glucuronic acid is found widely in plants and animals. One of its functions is to detoxify poisonous HO-containing compounds by reacting with them in the liver to form glucuronides. Glucuronides are water soluble and, therefore, readily excreted. After ingestion of a poison such as turpentine or phenol, the glucuronides of these compounds are found in urine. Draw the structure of the glucuronide formed by the reaction of beta-d-glucuronic acid and phenol.arrow_forwardWhat is the product of the starting material D-glyceraldehyde which will (1) produce aldaric acid upon reacting with HNO3 + H2O, NaOCH2, NH2OH, and (CH3CO)2O + NaOCOCH3 (2) produce tartaric acid upon reacting with HNO3 + H2O, NaOCH3, NH2OH, and (CH3CO)2O + NaOCOCH3arrow_forward
- Naturally occurring compounds called cyanogenic glycosides, such as lotaustralin, release hydrogen cyanide, HCN, when treated with aqueous acid. The reaction occurs by hydrolysis of the acetal linkage to form a cyanohydrin, which then expels HCN and gives a carbonyl compound. (a) Show the mechanism of the acetal hydrolysis and the structure of the cyanohydrin that results. (b) Propose a mechanism for the loss of HCN, and show the structure of the carbonyl compound that forms.arrow_forwardTreatment of -D-glucose with methanol in the presence of an acid catalyst converts it into a mixture of two compounds called methyl glucosides (Section 25.3A). In these representations, the six-membered rings are drawn as planar hexagons. (a) Propose a mechanism for this conversion and account for the fact that only the OH on carbon 1 is transformed into an OCH3 group. (b) Draw the more stable chair conformation for each product. (c) Which of the two products has the chair conformation of greater stability? Explain.arrow_forwardFats can be either optically active or optically inactive, depending on their structure. Draw the structure of an optically active fat that yields 2 equivalents of stearic acid and 1 equivalent of oleic acid on hydrolysis. Draw the structure of an optically inactive fat that yields the same products.arrow_forward
- Aspartame, the sweetener used in the commercial products NutraSweet and Equal, is 200 times sweeter than sucrose. What products will be obtained ifaspartame is hydrolyzed completely in an aqueous solution of HCl?arrow_forwardIllustrate the treatment of methyl α-D-glucopyranoside with aqueous acid forms a mixture of α- and β-D-glucose and methanol ?arrow_forwardd-Glucuronic acid is found widely in plants and animals. One of its functions is to detoxify poisonous HO-containing compounds by reacting with them inthe liver to form glucuronides. Glucuronides are water soluble and, therefore, readily excreted. After ingestion of a poison such as turpentine or phenol, theglucuronides of these compounds are found in urine. Draw the structure of the glucuronide formed by the reaction of β-d-glucuronic acid and phenol.arrow_forward
- Look up the structure of lisdexamfetamine (Vyvanse), a drug used in the treatment of attention deficit hyperactivity disorder (ADHD). Redraw it and identify all the functional groups present. What is known about itstherapeutic properties?arrow_forwardRing-A is considered a deoxy-pyranoside because it is missing an alcohol functional group on which carbon of ring-A? Use standard carbohydrate numbering. E в он он Oleandrin is a toxic cardiac glycoside found in the poisonous plant, oleander (Nerium oleander L). It has a very long IUPAC name: acetic acid [(35,5R, 10S,13R,14S,16S,17'R)-14-hydroxy-3-[[(2R,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyl- 2-tetrahydropyranyl]oxy]-10,13-dimethyl-17-(5-oxo-2H-furan-3-y)-1,2,3,4,5,6,7,8,9,11,12,15,16,17- tetradecahydrocyclopenta[a]phenanthren-16-yl] ester B.arrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



