
As we saw in Section 21.2, the reduction of iron oxides is accomplished by using carbon monoxide as a reducing agent. Starting with coke in a blast furnace, the following equilibrium plays a key role in the extraction of iron:
Use the data in Appendix 2 to calculate the equilibrium constant at 25°C and 1000°C. Assume ΔH° and ΔS° to be independent of temperature.
Appendix 2
Inorganic Substances
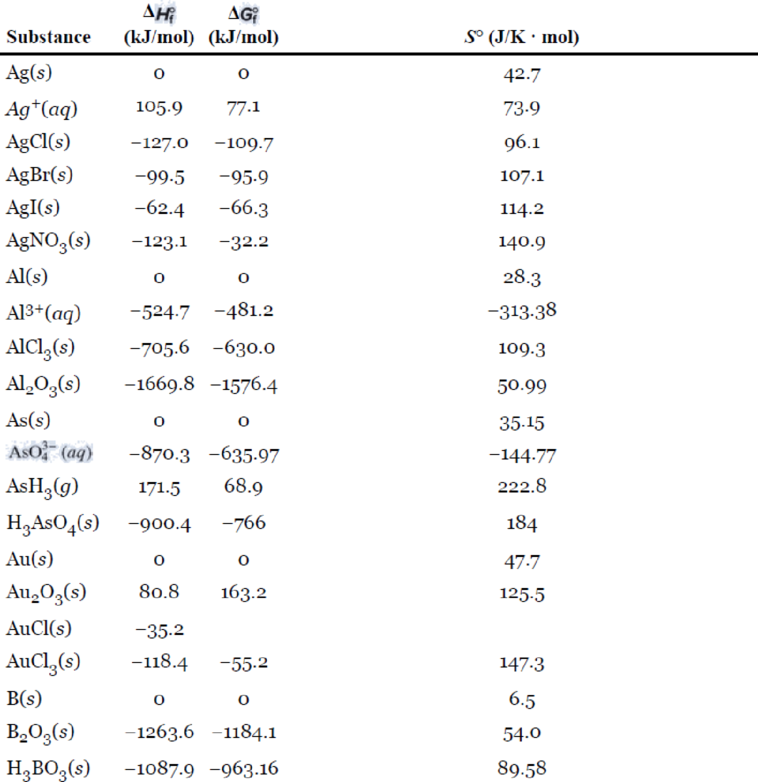
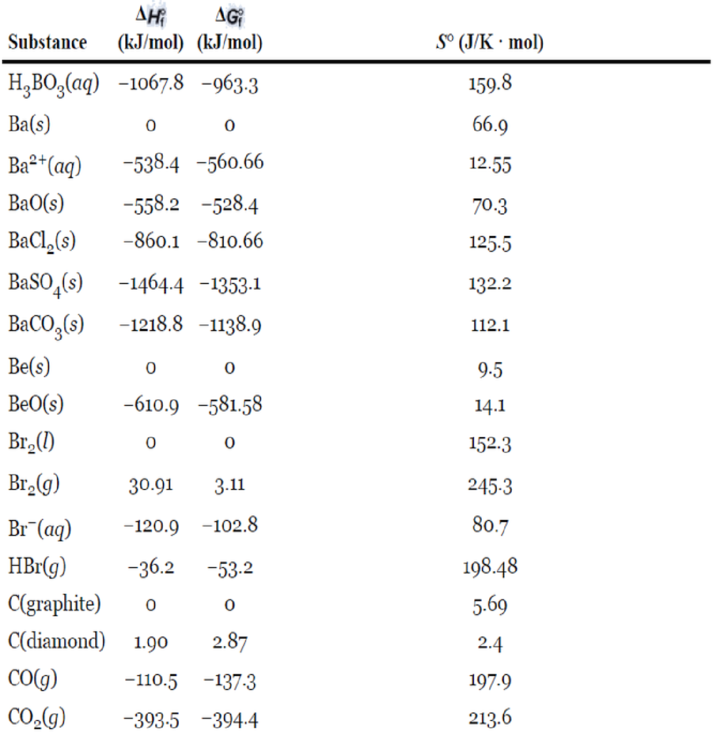
Interpretation:
For the given reaction, the equilibrium constant at
Concept Introduction:
Equilibrium constant:
Equilibrium constant is the ratio of the concentration of product and the concentration of the reactant at equilibrium.
It can be calculated using the relationship between Gibb’s free energy and rate constant:
Where,
Gibbs free energy: Gibbs free is a thermodynamic parameter, which can be defined as the amount of energy available with the system to perform a useful work.
Gibbs free energy Equation:
Where,
T– is Temperature in K.
The change in standard entropy can be calculated as follows:
The change in standard enthalpy can be calculated as follows:
Answer to Problem 22.104QP
For the given reaction,
The equilibrium constant at
The equilibrium constant at
Explanation of Solution
The given reaction is:
The following table gives the data of standard enthalpy of formation and standard entropy values for the reactants and products which are extracted from the appendix 2.
| Reactant/Product | Substance | ||
| Reactant | 0 | 5.69 | |
| Reactant | -393.5 | 213.6 | |
| Product | -110.5 | 197.9 |
Converting the temperature from
The change in standard enthalpy can be calculated as follows:
Converting it into
The change in standard entropy can be calculated as follows:
Calculating the Gibb’s free energy at
Calculating the equilibrium constant for the given reaction at
Thus, the equilibrium constant at
Calculating the Gibb’s free energy at
Calculating the equilibrium constant for the given reaction at
Thus, the equilibrium constant at
For the given reaction, the equilibrium constants at
Want to see more full solutions like this?
Chapter 22 Solutions
Chemistry
- Use data from Appendix J to calculate the enthalpy change and the Gibbs free energy change for the reduction of chromium(III) oxide by aluminum.arrow_forwardActually, the carbon in CO2(g) is thermodynamically unstable with respect to the carbon in calcium carbonate(limestone). Verify this by determining the standardGibbs free energy change for the reaction of lime,CaO(s), with CO2(g) to make CaCO3(s).arrow_forwardThe following data were collected for the reaction, H2(g) + L(g) ** - HI(g), at equilibrium at 25°C: [HJ = 0.10 mol L-1, [IJ = 0.20 mol L"\ [HI] = 4.0 mol L’1 Calculate the equilibrium constant for the reaction at this temperature.arrow_forward
- Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2 SO2 (g) + O2 (g) --> 2 SO3 (g)arrow_forwardThe standard free energy of formation of nitric oxide, NO, at 1000. K (roughly the temperature in an automobile engine during ignition) is 78.5 kJ/mol. Calculate the equilibrium constant for the reactionN2(g) + O2(g) 2NO(g)at 1000. K. a. 0.948 b. 1.57 × 105 c. 7.93 × 10–5 d. 6.29 × 10-9 e. -14.8arrow_forwardUsing the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: SnO2(s)+2CO(g)→Sn(s)+2CO2(g)arrow_forward
- Calcium metal is obtained by direct electrolysis of molten CaCl2. If a metallurgical electrolysis apparatus operates at 24.30 A, how many grams of calcium metal will be produced in 72 hours?arrow_forwardIs there any evidence that the combination of Cu2+ and Fe3+ is a more effective catalyst than either alone?arrow_forwardHydrazine, N2H4, has been proposed as the fuel in a fuel cell in which oxygen is the oxidizing agent. The reactions are N2H4(aq) + 4 OH(aq) N2(g) + 4 H2O() + 4e O2(g) + 2 H2O() + 4e 4 OH(aq) (a) Which reaction occurs at the anode and which at thecathode? (b) What is the overall cell reaction? (c) If the cell is to produce 0.50 A of current for 50.0 h, calculate what mass in grams of hydrazine must be present. (d) Calculate what mass (g) of O2 must be available to reactwith the mass of N2H4 determined in part (c).arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





