
Concept explainers
(a)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
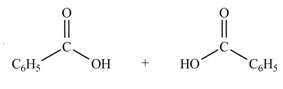
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield two equivalents of carboxylic acids, when they are treated with
The products formed by the treatment of benzoic anhydride
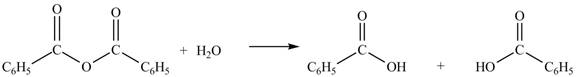
Figure 1
The products formed by the treatment of benzoic anhydride
(b)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
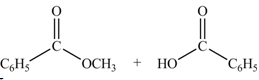
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield esters, and carboxylic acids, when they are treated with
The products formed by the treatment of benzoic anhydride
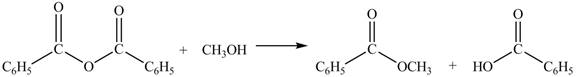
Figure 2
The products formed by the treatment of benzoic anhydride
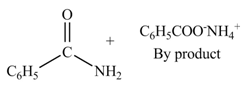
(c)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield amide and an ammonium salt of carboxylic acid, when they are treated with
The products formed by the treatment of benzoic anhydride
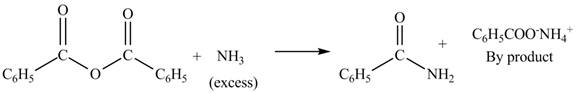
Figure 3
The products formed by the treatment of benzoic anhydride
(d)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
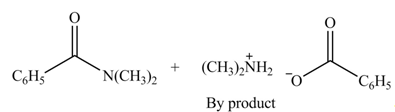
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield amide and an ammonium salt of carboxylic acid, when they are treated with
The products formed by the treatment of benzoic anhydride
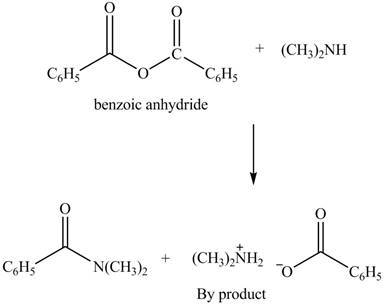
Figure 4
The products formed by the treatment of benzoic anhydride
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY(LL)W/ACCESS>CUSTOM<
- Draw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. a. HNO3, H2SO4 h. product in (a), then Sn, HClarrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. c. CH3CH2Cl, AlCl3 l. product in (c), then KMnO4arrow_forwardWhat product is formed when D-Gulose is treated with a. CH3I, Ag2O b. The product in (a), then H3O+ c. The product in (b), then C6H5CH2Cl, Ag2Oarrow_forward
- Nitromethane is reacted with ethyl prop-2-enoate with EtO-Na+, EtOH 3 equivalents to give the product X(C16). X then reacts with H2/Raney Ni to give the product Y(C14), which in turn reacts with Na to give Z (C12). Indicate which products X, Y and Z are.arrow_forwardWhen 2-pentene is treated with Cl2 in methanol, three products are formed. Account for the formation of each product (you need not explain their relative percentages).arrow_forwardWrite the main products when (i) n-butyl chloride is treated with alcoholic KOH. (ii) 2, 4, 6-trinitrochlorobenzene is subjected to hydrolysis. (iii) methyl chloride is treated with AgCN.arrow_forward
- Draw a stepwise mechanism for the conversion of acrylonitrile (CH2 = CHC ≡ N) to polyacrylonitrile, –[CH2CHC ≡ N]n–, using butyllithium (BuLi) as the initiator and CO2 as the electrophile to terminate the chain.arrow_forward6. Aldehydes are characterized by reactions: A) Nucleophilic addition of amines B) Nucleophilic addition of water C) Nucleophilic addition of alcohols D) Polymerizationarrow_forwardOximene and myrcene, two hydrocarbons isolated from alfalfa that have the molecular formula C10H16, both yield 2,6- dimethyloctane when treated with H2 and a Pd catalyst. Ozonolysis of oximene forms (CH3)2C = O, CH2 = O, CH2(CHO)2, and CH3COCHO. Ozonolysis of myrcene yields (CH3)2C = O, CH2 = O, (two equiv), and HCOCH2CH2COCHO. Identify the structures of oximene and myrcene.arrow_forward
- a) Draw two different enol tautomers of 2-methylcyclohexanone. (b) Draw two constitutional isomers that are not tautomers, but contain a C = C and an OH group.arrow_forwardproduct formed by treating butanal with 1. AgNO3 in HH3 2. Acidified K2CrO4 3. LiAlH4arrow_forwardExplain why methyl trifluoroacetate, CF3CO2CH3, is more reactive than methyl acetate, CH3CO2CH3, in nucleophilic acyl substitution reactions.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

