
ORGANIC CHEMISTRY-OWL V2 ACCESS
8th Edition
ISBN: 9781305582422
Author: Brown
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 22.50P
Following is the structure of miconazole, the active antifungal agent in a number of over-the-counter preparations, including Monistat, that are used to treat vaginal yeast infections. One of the compounds needed for the synthesis of miconazole is the trichloro derivative of toluene shown on its right.
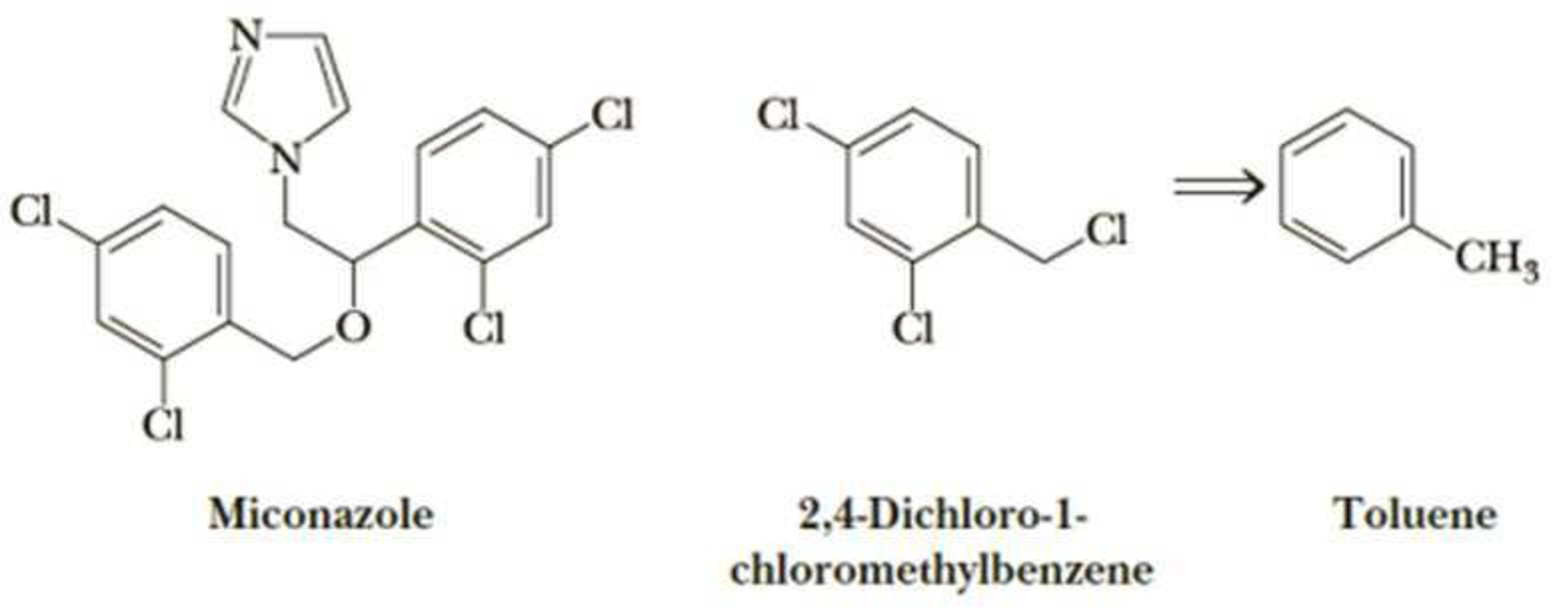
- (a) Show how this derivative can be synthesized from toluene.
- (b) How many stereoisomers are possible for miconazole?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider the tetracyclic aromatic compound drawn below, with rings labeled as A, B, C, and D. (a) Which of the four rings is most reactive in electrophilic aromatic substitution? (b) Which of the four rings is least reactive in electrophilic aromatic substitution? (c) What are the major product(s) formed when this compound is treated with one equivalent of Br2?
Consider the tetracyclic compound with rings labeled A–D. (a) Which ring is the most reactive in electrophilic aromatic substitution? (b) Which ring is the least reactive in electrophilic aromatic substitution?
4) Aromatic compounds are among the most abundant and versatile in nature. From a synthetic point of view, these compounds, despite their stabilities, are quite useful and can undergo reactions under special conditions and by specific mechanisms, such as the Electrophilic Aromatic Substitution (SAE) and the Nucleophilic Aromatic Substitution (SNAr). Based on this, please answer the following items:
(a) What are the possible isomeric products for the following reaction? Which structure, A or B, do you expect to predominate? Justify your choice. Write down the detailed mechanism of formation of compounds A and B. What would be the bromination product of each (compounds C and D)?
Chapter 22 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
Ch. 22.1 - Prob. 22.1PCh. 22.1 - Write a structural formula for the product from...Ch. 22.1 - Prob. 22.3PCh. 22.2 - Prob. 22.4PCh. 22.2 - Predict the major produce(s) of each electrophilic...Ch. 22.3 - In SN2 reactions of haloalkanes, the order of...Ch. 22 - Prob. 22.8PCh. 22 - Prob. 22.9PCh. 22 - Addition of m-xylene to the strongly acidic...Ch. 22 - Addition of tert-butylbenzene to the strongly...
Ch. 22 - What product do you predict from the reaction of...Ch. 22 - Other groups besides H+ can act as leaving groups...Ch. 22 - Prob. 22.14PCh. 22 - Prob. 22.15PCh. 22 - Prob. 22.16PCh. 22 - Prob. 22.17PCh. 22 - Suggest a reason why the nitroso group, N=O, is...Ch. 22 - Prob. 22.19PCh. 22 - Prob. 22.20PCh. 22 - The following molecules each contain two aromatic...Ch. 22 - Prob. 22.22PCh. 22 - Prob. 22.23PCh. 22 - The insecticide DDT is prepared by the following...Ch. 22 - Prob. 22.25PCh. 22 - Prob. 22.26PCh. 22 - Prob. 22.27PCh. 22 - Prob. 22.28PCh. 22 - Prob. 22.29PCh. 22 - Prob. 22.32PCh. 22 - Show how to prepare each compound from...Ch. 22 - Prob. 22.34PCh. 22 - Show reagents and conditions to bring about the...Ch. 22 - Prob. 22.36PCh. 22 - Propose a synthesis for each compound from...Ch. 22 - The first widely used herbicide for the control of...Ch. 22 - The first widely used herbicide for the control of...Ch. 22 - Prob. 22.40PCh. 22 - Prob. 22.41PCh. 22 - Prob. 22.42PCh. 22 - Prob. 22.43PCh. 22 - Cancer of the prostate is the second leading cause...Ch. 22 - Prob. 22.45PCh. 22 - Prob. 22.46PCh. 22 - Prob. 22.47PCh. 22 - When certain aromatic compounds are treated with...Ch. 22 - Prob. 22.49PCh. 22 - Following is the structure of miconazole, the...Ch. 22 - Prob. 22.51PCh. 22 - Prob. 22.52PCh. 22 - Prob. 22.53PCh. 22 - Show how the antidepressant venlafaxine (Effexor)...Ch. 22 - Prob. 22.57PCh. 22 - Given this retrosynthetic analysis, propose a...Ch. 22 - Prob. 22.59PCh. 22 - Prob. 22.60PCh. 22 - Prob. 22.61PCh. 22 - A newer generation of antipsychotics, among them...Ch. 22 - Prob. 22.63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The bicyclic heterocycles quinoline and indole undergo electrophilic aromatic substitution to give the products shown. (a) Explain why electrophilic substitution occurs on the ring without the N atom for quinoline, but occurs on the ring with the N atom in indole. (b) Explain why electrophilic substitution occurs more readily at C8 than C7 in quinoline. (c) Explain whyelectrophilic substitution occurs more readily at C3 rather than C2 of indole.arrow_forwardThe hydrocarbon fluorene was treated with potassium t-butoxide in an acid-base reaction, giving the fluorenide anion and t-butyl alcohol. (a) Which way does the equilibrium lie, and by how much? b) What is the proportion of the fluorenide anion to fluorene? (c) Why is fluorene so highly acidic, considering the pKa of an average alkane is above 50?arrow_forward11:43 Q1. (a) (c) (d) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide. (i) Explain what is meant by the term stereoisomers. Library Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH3)2C=CH₂ Name of mechanism Mechanism (ii) Draw the structures and give the names of the two stereoisomers of but-2-ene. Stereoisomer 1 Name (iii) Name this type of stereoisomerism. Select Name Stereoisomer 2 When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. CH3 H₂C-C-CH3 + KOH Br Page 2 of 14 CH3 H3C-C-CH3 + KBr ОН State the role of the hydroxide ions in this reaction. Write an equation for the reaction that occurs when CH3CH₂CH₂CH₂Br reacts with an excess of ammonia. Name the organic product of this reaction. Equation Name of product 9,284 Photos, 1,166 Videos For You…arrow_forward
- (a) Which of the following will NOT produce a carboxylic acid or carboxylate ion? 1-butanol + H2CrO4 2-butene + O3/H2O2 butanal + PCC butanal + H2CrO4 (b) Which of the following will NOT produce a carboxylic acid or carboxylate ion? 1-butanol + H2CrO4 2-butene + O3/H2O2 butanal + PCC butanal + H2CrO4arrow_forwardCompound A has molecular formula C7H15B.. Treatment of compound A with sodium ethoxide yields only one elimination product (compound B) and no substitution products. When compound B is treated with dilute sulfuric acid, compound C is obtained, which has molecular formula C7H160. Draw the structures of compounds A, B, and C.arrow_forwardConsider the tetracyclic aromatic compound drawn below, with rings labeled as A, B, C, and D. (a) Which of the four rings is most reactive in electrophilic aromatic substitution? (b) Which of the four rings is least reactive in electrophilic aromatic substitution? (c) What are the major product(s) formed when this compound is treated with one equivalent of Br2?arrow_forward
- Give each of the following structures an IUPAC name. (a) (b) H3C H3C² Н2 ОН HC. H2 Н, ОН CH3 (c) (d) НО НО Н, Н, CH ОН C H2 ОН НС. OH CH3arrow_forwardCompound A exhibits a peak in its 1H NMR spectrum at 7.6 ppm, indicating that it is aromatic. (a) How are the carbon atoms of the triple bonds hybridized? (b) In what type of orbitals are the π electrons of the triple bonds contained? (c) How many π electrons are delocalized around the ring in A?arrow_forwardExplain the following behaviours :(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol.arrow_forward
- Gg.108.arrow_forwardWhich of the following are aromatic?arrow_forward(a) How will you convert:(i) Benzene to acetophenone (ii) Propanone to 2-Methylpropan-2-ol(b) Give reasons :(i) Electrophilic substitution in benzoic acid takes place at meta position.(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY