
(a)
Interpretation:
The given reaction is to be completed.
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.88AP
The complete reaction is shown below.
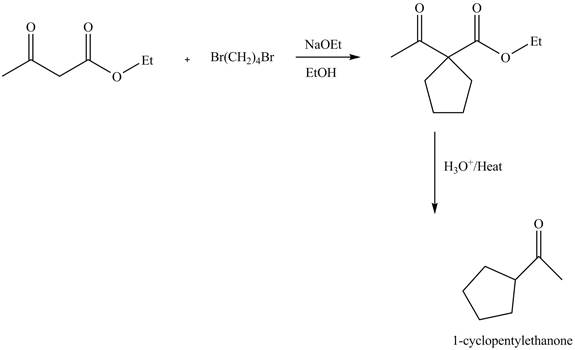
Explanation of Solution
The reaction of ethyl acetoacetate with dibromobutane in presence of base and ethanol results in the dialkylation reaction. It forms a cyclic product. This product undergoes hydrolysis reaction followed by decarboxylation reaction gives
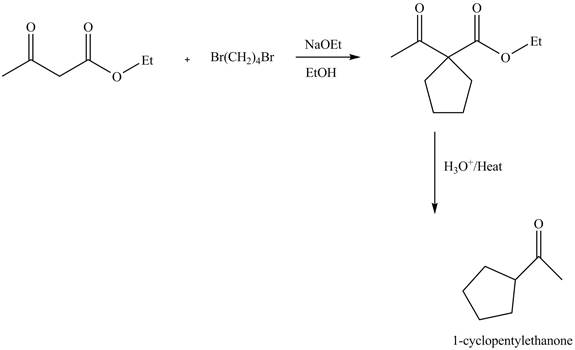
Figure 1
The complete reaction is shown in Figure 1.
(b)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Lactones are cyclic carboxylic esters analogous to one heteroatom cyclic ring. It is formed by intramolecular esterification reactions. Generally, five of six members lactones are more reactive. Lithium isopropylamine is a strong, non-nucleophilic base.
Answer to Problem 22.88AP
The complete reaction is shown below.
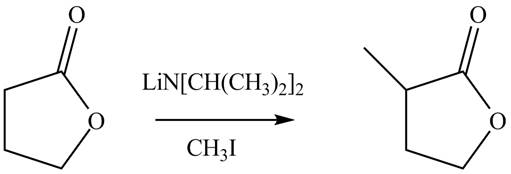
Explanation of Solution
The reaction of lactone which is a cyclic ester with lithium isopropylamide which is used as a base to abstract a proton. It forms an enolate ion which attacks a electrophile methyl iodide. It result in the formation of methyl substituted lactone compound. The given reaction is completed as shown below.
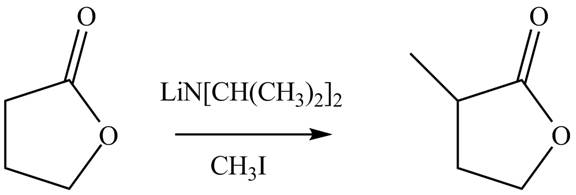
Figure 2
The complete reaction is shown Figure 2.
(c)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Aryl halides undergo substitution reactions only under drastic conditions. They generally are of two types addition-elimination and elimination reactions. The elimination addition reaction involves a benzyne intermediate. Whereas, in addition, an elimination reaction involves Meisenheimer complex formation.
Answer to Problem 22.88AP
The complete reaction is shown below.
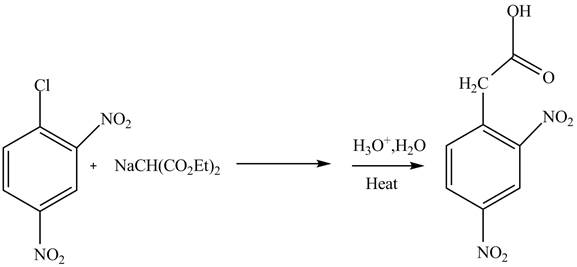
Explanation of Solution
The reaction of
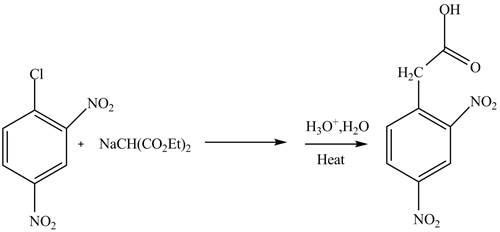
Figure 3
The complete reaction is shown in Figure 3.
(d)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The reduction of carbonyl compound is carried out by different reagents. For example
Answer to Problem 22.88AP
The complete reaction is shown below.
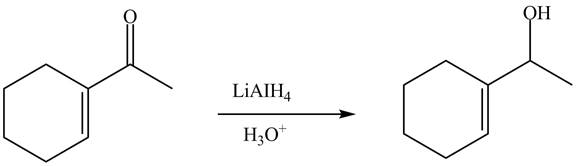
Explanation of Solution
The reaction of
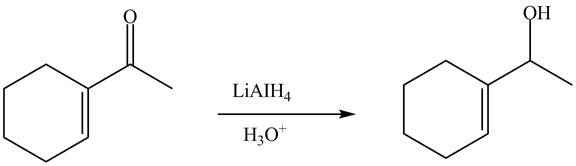
Figure 4
The complete reaction is shown in Figure 4.
(e)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Michael addition reaction is a nucleophilic addition reaction of an anion to
Answer to Problem 22.88AP
The complete reaction is shown below.
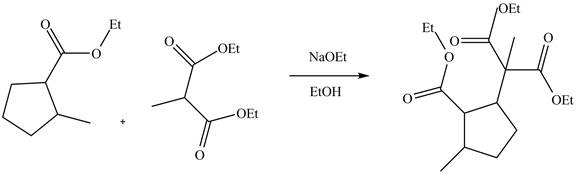
Explanation of Solution
The reaction of an ester with a base result in the abstraction of an acidic proton and form an enolate ion. This enolate ion undergoes

Figure 5
The complete reaction is shown in Figure 5.
(f)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The Knoevenagel condensation reaction is a modification of aldol condensation reaction. In this nucleophilic hydrogen atom adds to carbonyl group followed by dehydration reaction. The compound obtained is known as
Answer to Problem 22.88AP
The complete reaction is shown below.
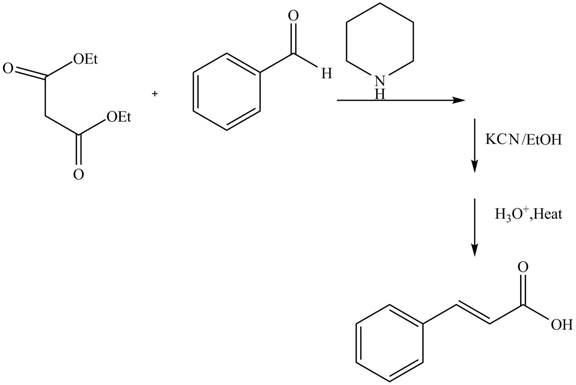
Explanation of Solution
The reaction of benzaldehyde and diethyl malonate in presence of piperdine base which forms an iminium intermediate ion. The reaction in presence of

Figure 6
The complete reaction is shown in Figure 6.
(g)
Interpretation:
The given reaction is to be completed.
Concept introduction:
An ester is a derivative of carboxylic which is obtained by replacing the
Answer to Problem 22.88AP
The complete reaction is shown below.
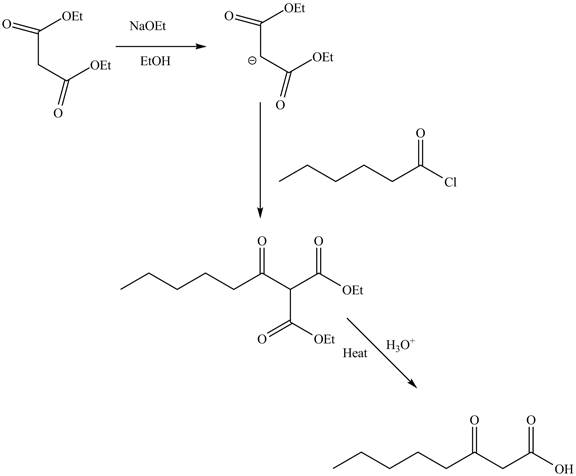
Explanation of Solution
The reaction of diethyl malonate in the presence of a base
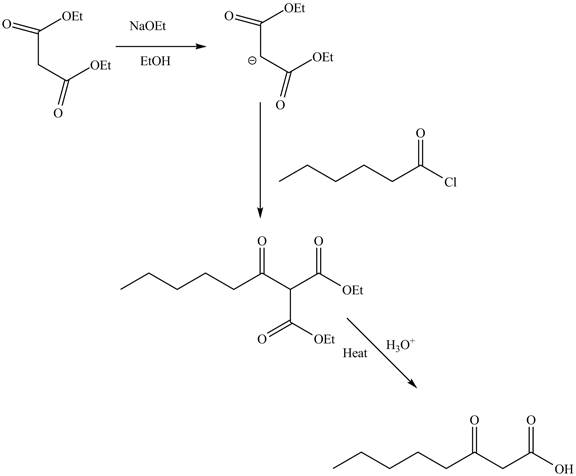
Figure 7
The complete reaction is shown in Figure 7.
(h)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Michael addition reaction is a nucleophilic addition reaction of an anion to
Answer to Problem 22.88AP
The complete reaction is shown below.
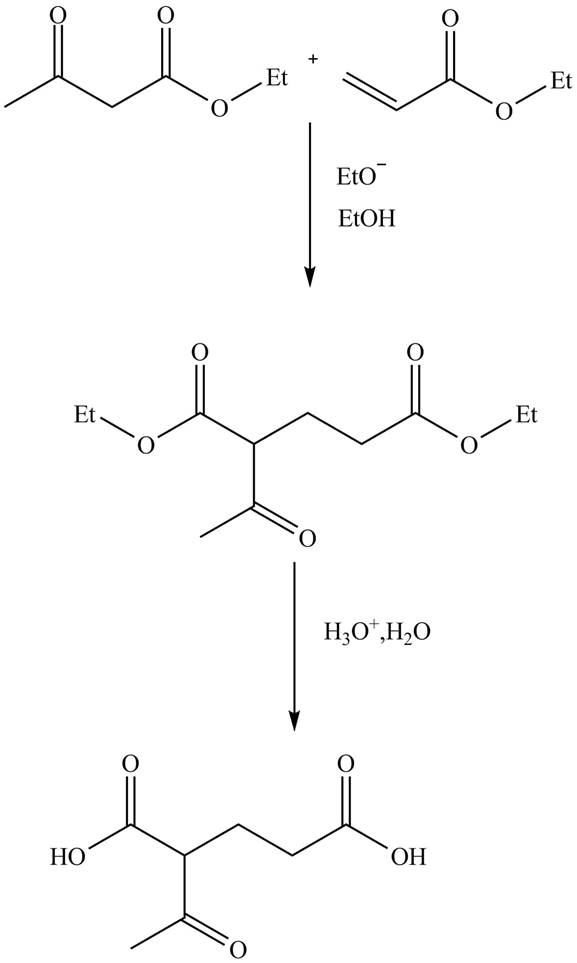
Explanation of Solution
The reaction of ethylacetoacetate with ethyl acrylate in presence of base
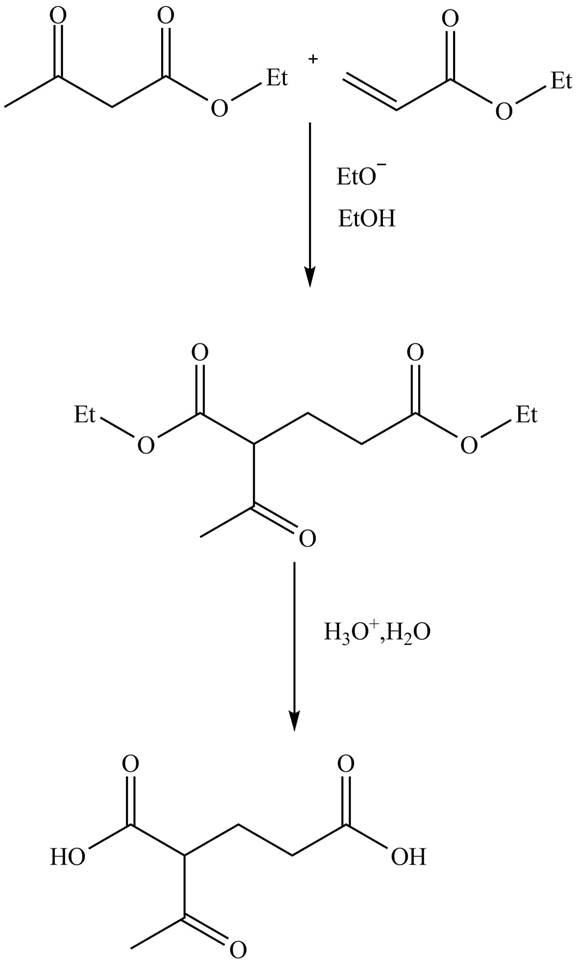
Figure 8
The complete reaction is shown in Figure 8.
(i)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Grignard reagents are
Answer to Problem 22.88AP
The complete reaction is shown below.
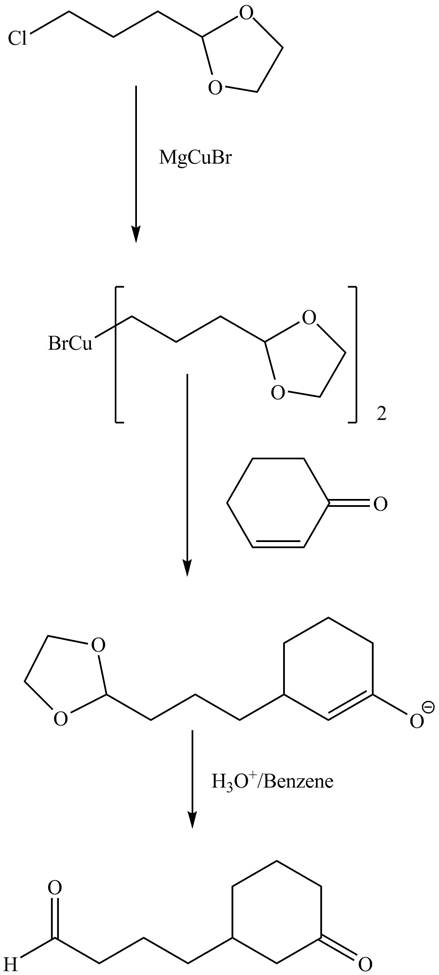
Explanation of Solution
The reaction of given dioxolane compound with
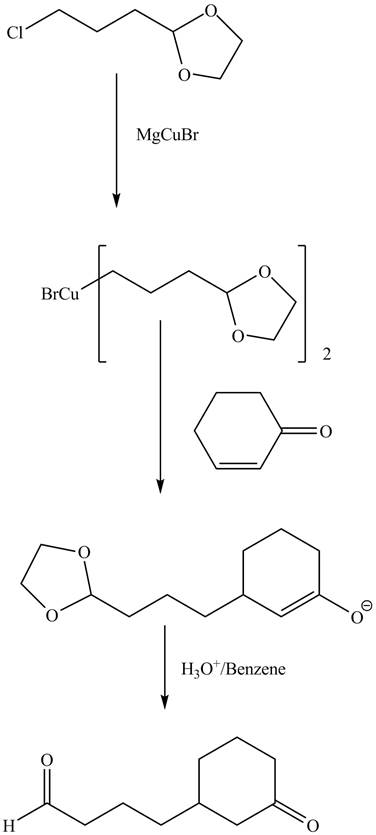
Figure 9
The complete reaction is shown in Figure 9.
(j)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases. Gilman reagent is a lithium and copper reagent. They react with organohalide to replace the halide group. They are used in Corey-House synthesis reaction.
Answer to Problem 22.88AP
The complete reaction is shown below.
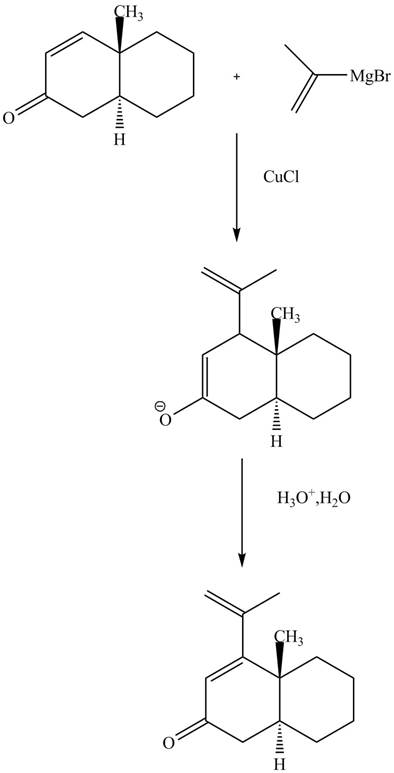
Explanation of Solution
The reaction of unsaturated Grignard reagent in the presence of
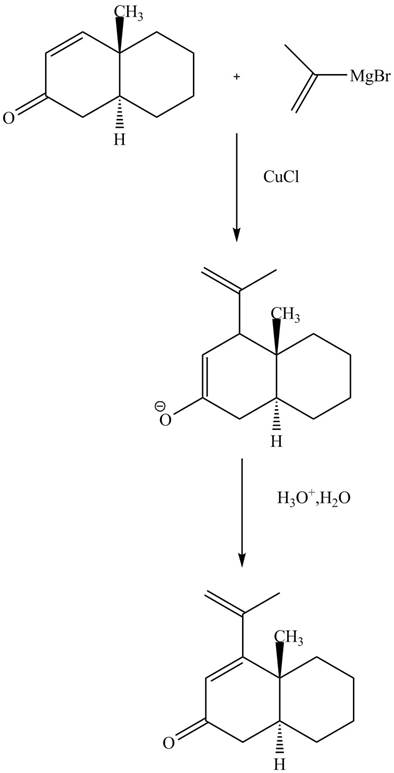
Figure 10
The complete reaction is shown in Figure 10.
(k)
Interpretation:
The given reaction is to be completed.
Concept introduction:
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases. Gilman reagent is a lithium and copper reagent. They react with organohalide to replace the halide group. They are used in Corey-House synthesis reaction.
Answer to Problem 22.88AP
The complete reaction is shown below.
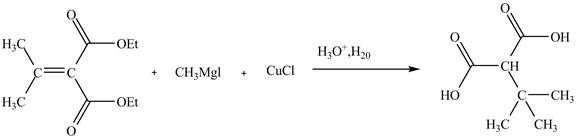
Explanation of Solution
The reaction of the Grignard reagent with
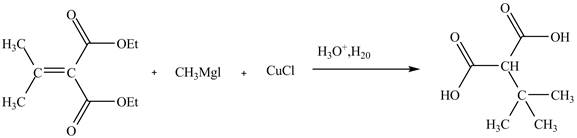
Figure 11
The complete reaction is shown in Figure 11.
(l)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The replacement of hydrogen atom attached to a carbon atom of electron-rich benzene ring by an incoming electrophile is known as electrophilic aromatic substitution reaction. The rate of electrophilic aromatic substitution reaction depends on the substituted group on the aromatic ring.
Answer to Problem 22.88AP
The complete reaction is shown below.
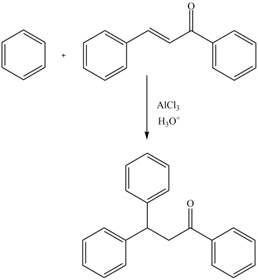
Explanation of Solution
The electrophilic substitution reaction of benzene with

Figure 12
The complete reaction is shown in Figure 12.
(m)
Interpretation:
The given reaction is to be completed.
Concept introduction:
The Claisen condensation reaction in which two esters or one ester and a carbonyl compound react together to form
Answer to Problem 22.88AP
The complete reaction is shown below.
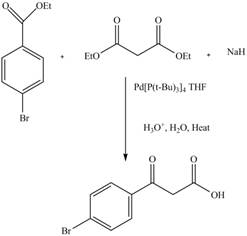
Explanation of Solution
The reaction of diethyl malonate in the presence a base forms an enolate ion. This attacked on another ester molecule and result in the formation of

Figure 13
The complete reaction is shown in Figure 13.
Want to see more full solutions like this?
Chapter 22 Solutions
Loose-leaf Version For Organic Chemistry
- Give a clear handwritten answer with explanation..give the detailed mechanism of given bleow reaction?arrow_forwardGive a clear handwritten answer with explanation needed!!! Give the mechanism...arrow_forwardGive the clear handwritten answer and give the mechanism of given bleow reactionsarrow_forward
- Give a clear handwritten answer with explanation needed...give the mechanism of given bleow reactions....give answer in detailed..?arrow_forwardGive the clear handwritten answer..and give synthesis mechanism of given bleow reactions...arrow_forwardGive a clear handwritten answer with explanation....give the products when given bleow structure reacts presence of giving each reagents ...arrow_forward
- Give a clear handwritten answer with explanation..give the mechanism of given bleow reaction with detailed answer..?arrow_forwardGive a clear handwritten answer with explanation. ..give below some options choose the in which given options who react with Tollen's reagent...give textual explanation also...?arrow_forwardGive a clear handwritten answer..with explanation..give the mechanism of given bleow reactionsarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





