
(a)
Interpretation:
A composition for each molecule and the expected isotopic peak intensities has to be suggested and calculated.
(a)
Explanation of Solution
Form the given intensity we can identified a compound for
The molecular formula for phenol is
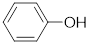
Now,
The expected intensity for
Observed intensity of
Expected intensity of
Observed intensity of
(b)
Interpretation:
A composition for each molecule and the expected isotopic peak intensities has to be suggested and calculated.
(b)
Explanation of Solution
Form the given intensity we can identified a compound for
The molecular formula for bromophenol is
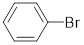
Now,
Here, h includes
The expected intensity for
Observed intensity of
Expected intensity of
Observed intensity of
The isotopic partner of
Hence, the expected intensity of
The predicted intensity of (
Observed intensity of
(c)
Interpretation:
A composition for each molecule and the expected isotopic peak intensities has to be suggested and calculated.
(c)
Explanation of Solution
From the given data
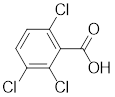
The molecular formula is
The expected intensity for
Observed intensity of
Expected intensity of
Observed intensity of
The
Expected intensity of
Predicted intensity of (
Expected intensity of
Observed intensity
For
Other formulas like
Expected intensity of
The contribution from
The predicted intensity of
The predicted intensity from
The total expected intensity of
Observed intensity
Expected intensity of
Observed intensity
(d)
Interpretation:
A composition for each molecule and the expected isotopic peak intensities has to be suggested and calculated.
(d)
Explanation of Solution
From the given data
The structure for this compound can be shown as follows.
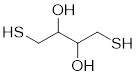
Now let’s find the following things,
The expected intensity for
Observed intensity of
Expected intensity of
Observed intensity of
Want to see more full solutions like this?
Chapter 22 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





