
(a)
Interpretation: The category of reaction from below substrates should be identified along with the product formed in the reaction and appropriate curved arrows should be used to show electron movement.
Concept introduction: The fundamental electrostatics suggests that electrons have more affinity for electron-deficient sites in an organic compound or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron-deficient species.
(b)
Interpretation: The wrong thing about below arrow diagrams should be indicated.
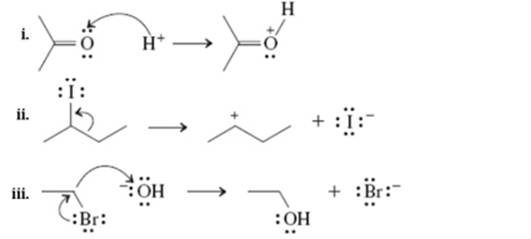
Concept introduction: The fundamental electrostatics suggests that electrons have more affinity for electron deficient sites in an organic compound or positive charge. The curved arrow is appropriate mechanism to depict electron movement that occurs from electron rich species to electron deficient species.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- You are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forwardUSING ARROWS TO DENOTE ELECTRON FLOW- PROVIDE a detailed reaction mechanism for the reaction scheme belowarrow_forwardCumene -> i. Butyl chloride ii. Dimethyl amine iii. H2, Pt Provide the bond-line structures for the major organic products obtained in each steparrow_forward
- . Discuss the truth of the following statement. Explain why it is true or false Every SN1 reaction produces racemic mixtures in the productsarrow_forwardPropose a reasonable mechanism for the reaction, drawing out Lewis structure, using arrows to show electron flow, clearly identifying the bond making/bond breaking steps, and proposing reasonable intermediates for the reaction.arrow_forwardgive the degree of unsaturation of the compounds: Vanillin(C8H8O3) and ethyl ethocyacetate (C6H12O3). and draw their structuresarrow_forward
- Outline the step-by-step method (initiation, propagation(s), and one termination step) for bromination of 2-bromopropane to produce its major product.arrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forwardThe compound whose structure is shown here is acetyl acetone. It exists in two forms:the enol form and the keto form The molecule reacts with OH–to form an anion, [CH3COCHCOCH3] (often abbreviatedacac–for acetylacetonate ion). One or the most interesting aspects of this anion is thatone or more of them can react with transition metal cations to give stable, highlycolored compounds (a) Are the keto and enol forms of acetylacetone resonance forms? Explain youranswer.(b) What is the hybridization or each atom (except H) in the enol form? What changesin hybridization occur when it is transformed into the keto form?(c) What are the electron-pair geometry and molecular geometry around each C atomin the keto and enol forms? What changes in geometry occur when the keto formchanges to the enol form?(d) Draw three possible resonance structures for the acac–ion.(e) Is cis-trans isomerism possible in either the enol or the keto form of acetylacetone?(f) Is the enol form of acetylacetone polar?…arrow_forward
- The German chemist Wilhelm Kӧrner (1839-1925) observed in 1974 that each of the three isomers of dibromobenzene, A, B, and C, gave a different number of tribromobenzenes upon further bromination, allowing him to assign their respective structures. Try to do the same and assign structures to A, B, and C based on the following results: A gives two tribromobenzenes in comparable amounts B gives three tribromobenzenes, one of them in minor quantities C gives only one tribromobenzenearrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst to give hydrocarbon B. Hydrocarbon A also reacts with OsO4 to give the glycol C. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2COOH, and the other fragment is ketone D (R2C=O). What are the structures of A, B, C and D? Write all reactions.arrow_forwardWrite structural formulas for toluene (C6H5CH3) and for benzoic acid (C6H5CO2H) (a) as resonance hybrids of two Kekulé forms and (b) with the Robinson symbol.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

