
Chemistry: Molecular Approach - Package
4th Edition
ISBN: 9780134577982
Author: Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 22, Problem 34E
Interpretation Introduction
Interpretation: The following fatty acid is most likely to be a solid at room temperature or not.
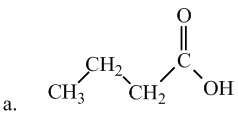
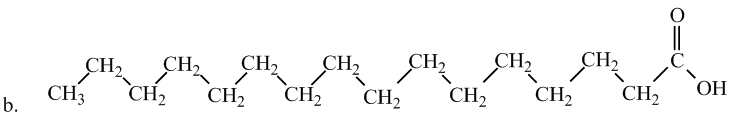
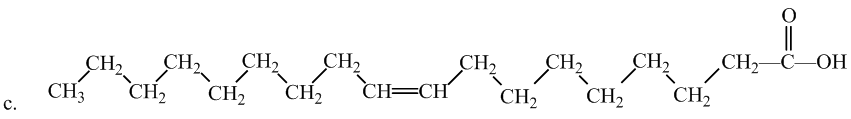
Concept Introduction:
Saturated fatty acids experience greater molecular forces, making them solid at room temperature. Therefore, option (b) is most likely to be a solid at room temperature. The hydrocarbon chains in this fatty acid are fairly straight and packed closely together, making it solid at room temperature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Chemistry: Molecular Approach - Package
Ch. 22 - Prob. 1SAQCh. 22 - Prob. 2SAQCh. 22 - Prob. 3SAQCh. 22 - Prob. 4SAQCh. 22 - Prob. 5SAQCh. 22 - Prob. 6SAQCh. 22 - Prob. 7SAQCh. 22 - Prob. 8SAQCh. 22 - Q9. Peptide bonds occur in which type of...Ch. 22 - Q10. How many nucleotides are required to code for...
Ch. 22 - 1. What is biochemistry? What significant advances...Ch. 22 - Prob. 2ECh. 22 - Prob. 3ECh. 22 - 4. What effect do double bonds have within the...Ch. 22 - Prob. 5ECh. 22 - Prob. 6ECh. 22 - 7. Describe the basic structure of phospholipids...Ch. 22 - 8. What is a steroid? List some functions of...Ch. 22 - Prob. 9ECh. 22 - Prob. 10ECh. 22 - Prob. 11ECh. 22 - Prob. 12ECh. 22 - Prob. 13ECh. 22 - Prob. 14ECh. 22 - Prob. 15ECh. 22 - Prob. 16ECh. 22 - Prob. 17ECh. 22 - Prob. 18ECh. 22 - Prob. 19ECh. 22 - Prob. 20ECh. 22 - Prob. 21ECh. 22 - Prob. 22ECh. 22 - Prob. 23ECh. 22 - Prob. 24ECh. 22 - Prob. 25ECh. 22 - Prob. 26ECh. 22 - 27. What is a codon? A gene? A chromosome?
Ch. 22 - Prob. 28ECh. 22 - Prob. 29ECh. 22 - Prob. 30ECh. 22 - Prob. 31ECh. 22 - Prob. 32ECh. 22 - Prob. 33ECh. 22 - Prob. 34ECh. 22 - 35. Draw structures showing the reaction of...Ch. 22 - Prob. 36ECh. 22 - Prob. 37ECh. 22 - Prob. 38ECh. 22 - Prob. 39ECh. 22 - Prob. 40ECh. 22 - Prob. 41ECh. 22 - Prob. 42ECh. 22 - Prob. 43ECh. 22 - Prob. 44ECh. 22 - Prob. 45ECh. 22 - Prob. 46ECh. 22 - Prob. 47ECh. 22 - Prob. 48ECh. 22 - 49. Draw each amino acid in its dipolar ion...Ch. 22 - 50. Draw each amino acid in its dipolar ion...Ch. 22 - Prob. 51ECh. 22 - Prob. 52ECh. 22 - Prob. 53ECh. 22 - Prob. 54ECh. 22 - Prob. 55ECh. 22 - Prob. 56ECh. 22 - Prob. 57ECh. 22 - Prob. 58ECh. 22 - 59. A phenylalanine amino acid on a protein strand...Ch. 22 - 60. An amino acid on a protein strand forms a...Ch. 22 -
61. The amino acid sequence in one section of a...Ch. 22 - Prob. 62ECh. 22 - Prob. 63ECh. 22 - Prob. 64ECh. 22 - Prob. 65ECh. 22 - Prob. 66ECh. 22 - Prob. 67ECh. 22 - Prob. 68ECh. 22 - Prob. 69ECh. 22 - Prob. 70ECh. 22 - 71. Determine the class of biochemical compound...Ch. 22 - Prob. 72ECh. 22 - 73. What is the difference between a codon and a...Ch. 22 - 74. What is the difference between a fatty acid...Ch. 22 - Prob. 75ECh. 22 - Prob. 76ECh. 22 - Prob. 77ECh. 22 - Prob. 78ECh. 22 - 79. Determining the amino acid sequence in a...Ch. 22 - Prob. 80ECh. 22 - Prob. 81ECh. 22 - 82. Calculate the mass percent of phosphorus in a...Ch. 22 - 83. The double helical structure of DNA disrupts...Ch. 22 - Prob. 84ECh. 22 - Prob. 85ECh. 22 - Prob. 86ECh. 22 - Prob. 87ECh. 22 - Prob. 88ECh. 22 - 89. Write the major equilibrium that is...Ch. 22 - Prob. 90ECh. 22 - Prob. 91ECh. 22 - 92. The genetic code is random, which means that a...Ch. 22 - Prob. 93QGWCh. 22 - Prob. 94QGWCh. 22 - Prob. 95QGWCh. 22 - Prob. 96QGWCh. 22 - Prob. 97QGWCh. 22 - Prob. 98DIA
Knowledge Booster
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY