
Concept explainers
Interpretation:
The synthesis of each of the given compound from the indicated starting material is to be stated.
Concept introduction:
The hydration of
The reagent
Lithium aluminum hydride and sodium borohydride are strong reducing agents. They are inorganic compounds and are used as the reducing agents in organic synthesis. They are used for the conversion of carboxylic acids, aldehydes and ketones into primary and secondary alcohols.
Answer to Problem 49P
Solution:
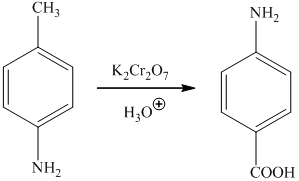
a) The synthesis of
b) The synthesis of
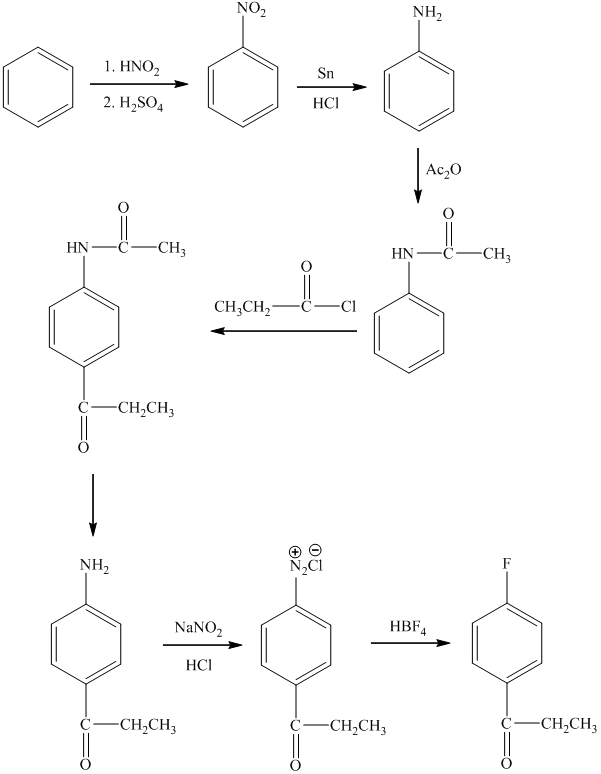
c) The synthesis of
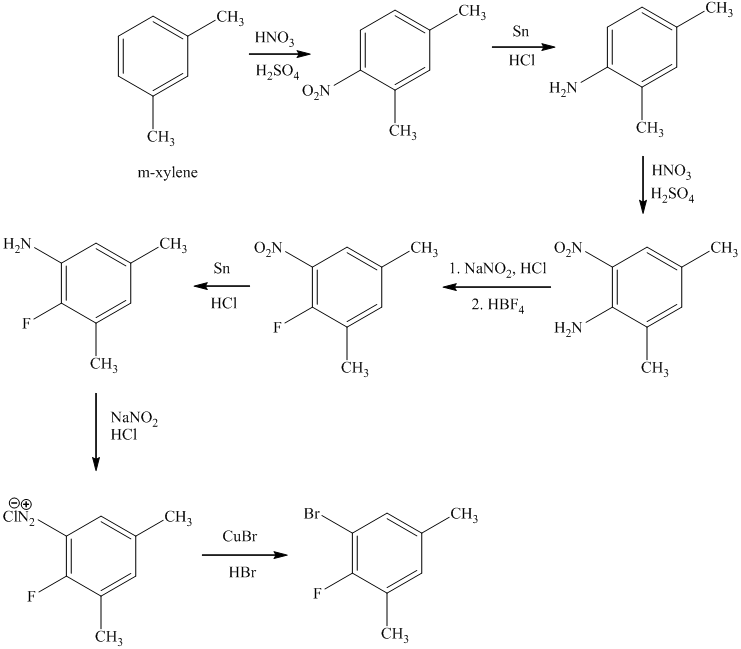
d) The synthesis of
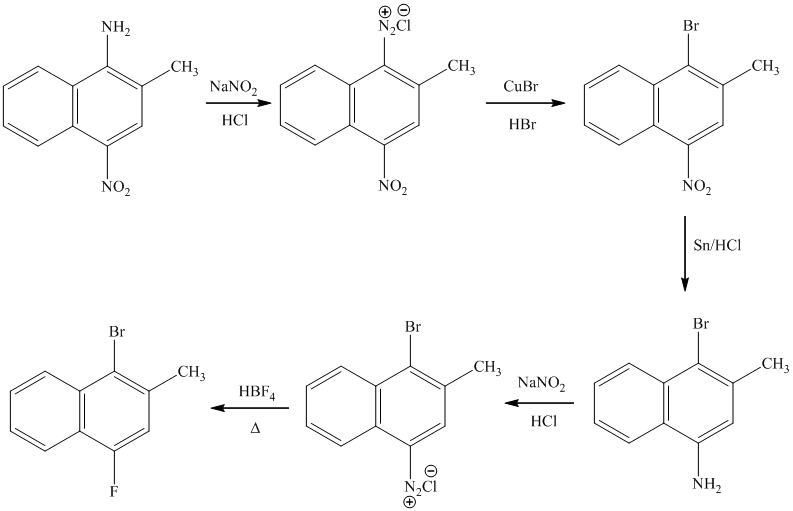
e) The synthesis of
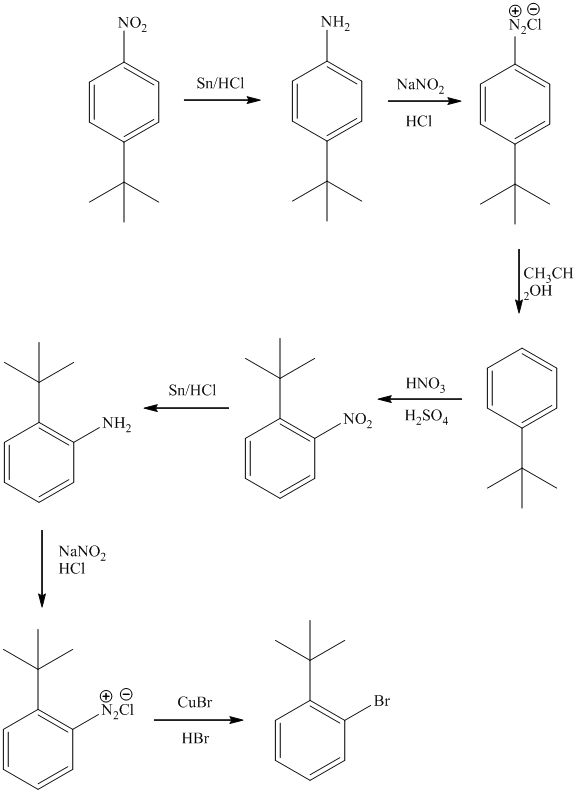
f) The synthesis of
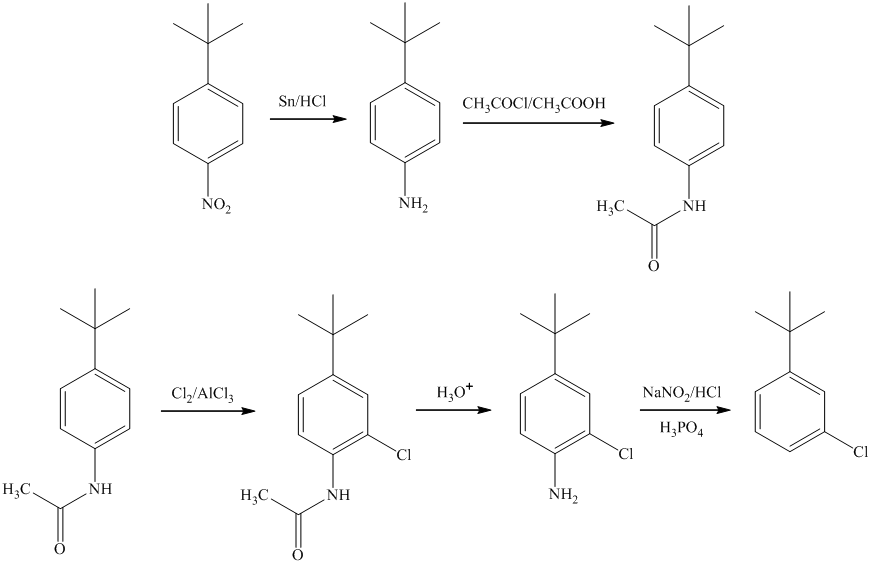
g) The synthesis of
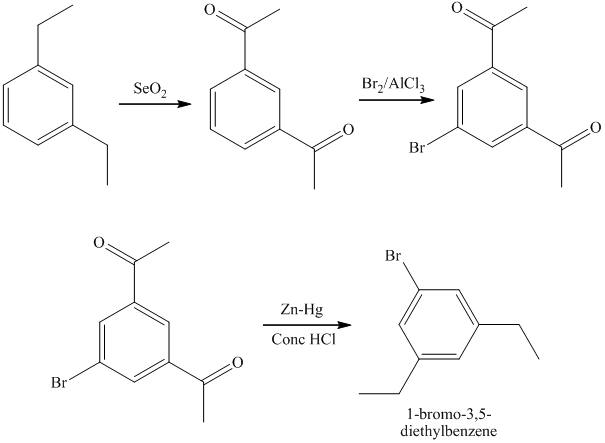
h) The synthesis of
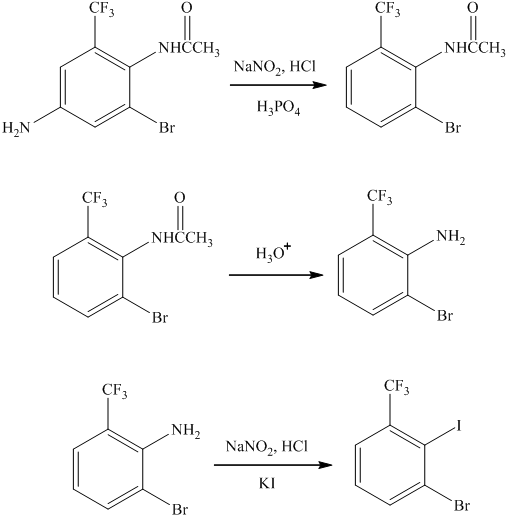
i) The synthesis of the desired compound from its starting material is shown below.
Explanation of Solution
a)
The preparation of

b)
In the preparation of
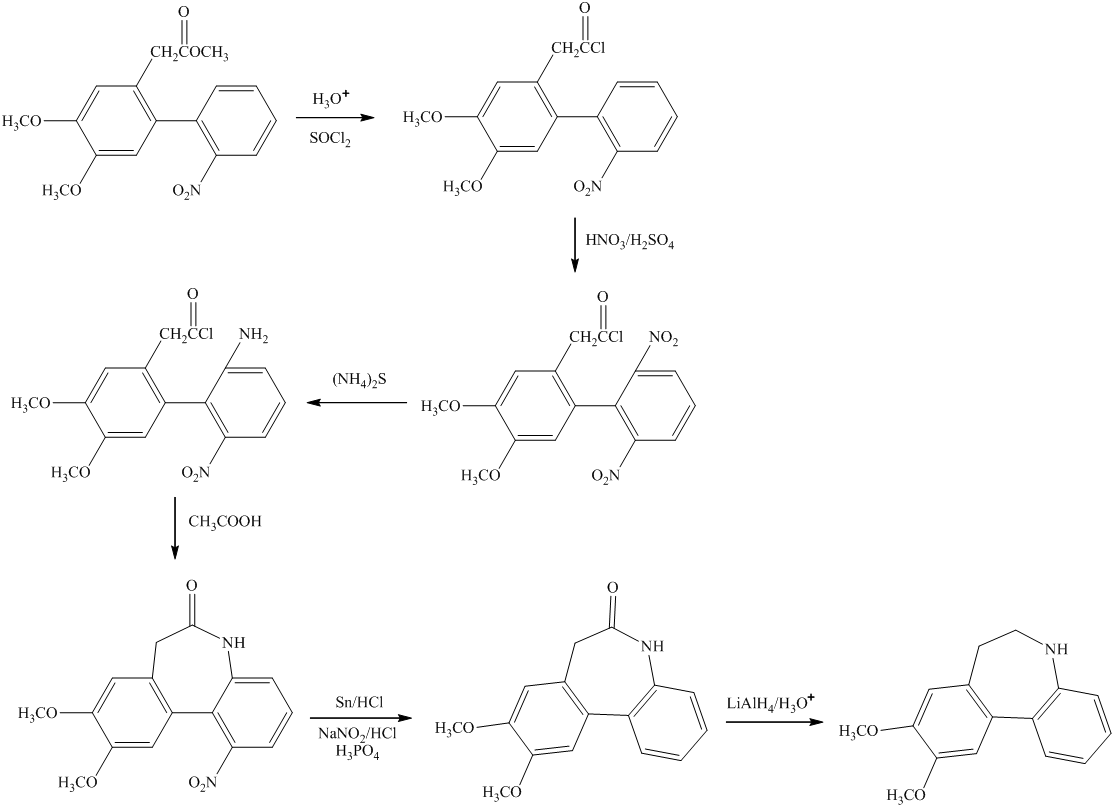
c)
In the preparation of
In the next step, further nitration takes place followed by the addition of
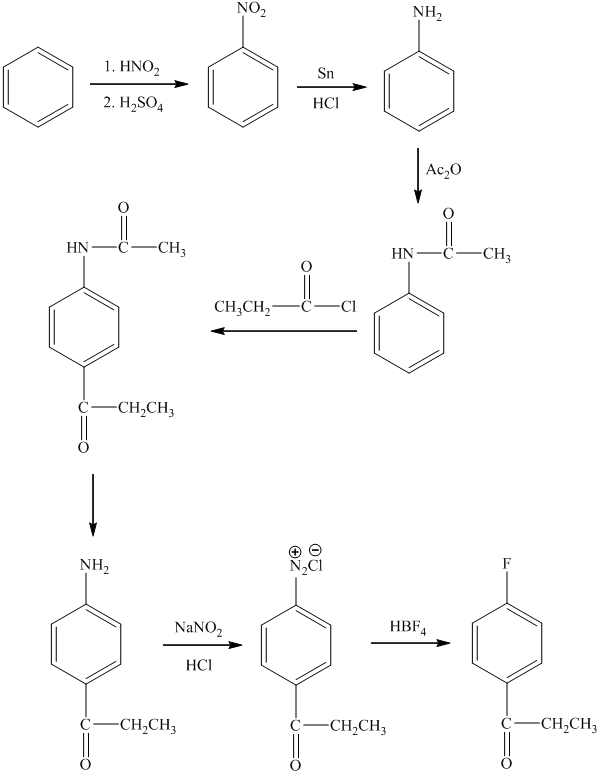
d)
In the first step of the reaction, the starting material reacts with
In the next step, the resulting compound reacts with
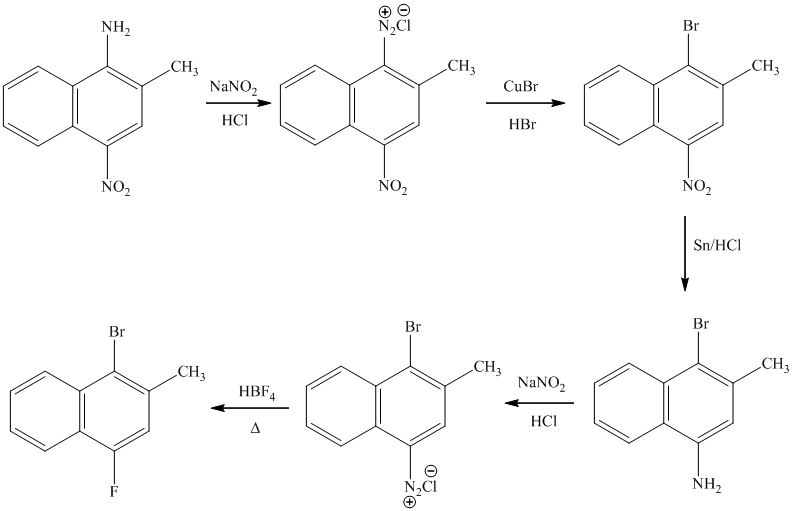
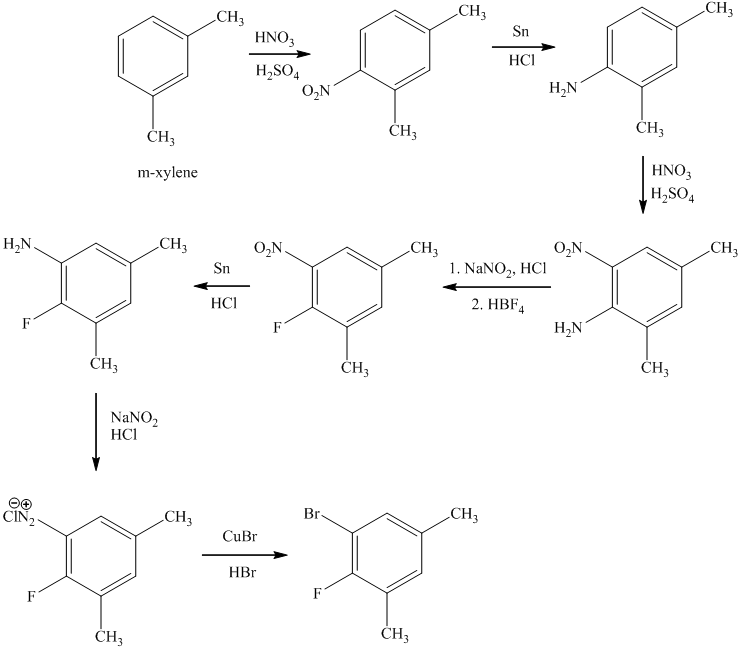
e)
The preparation of
In the next step, the resulting compound reacts with
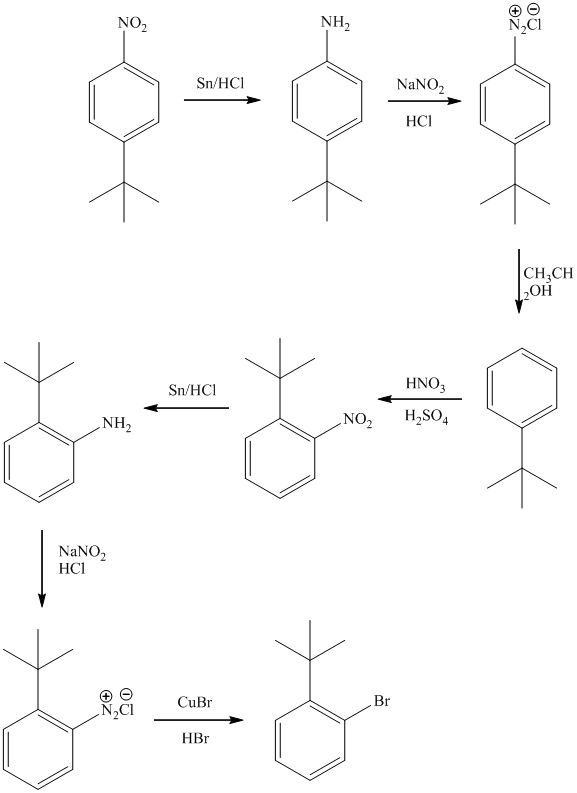
f)
The preparation of
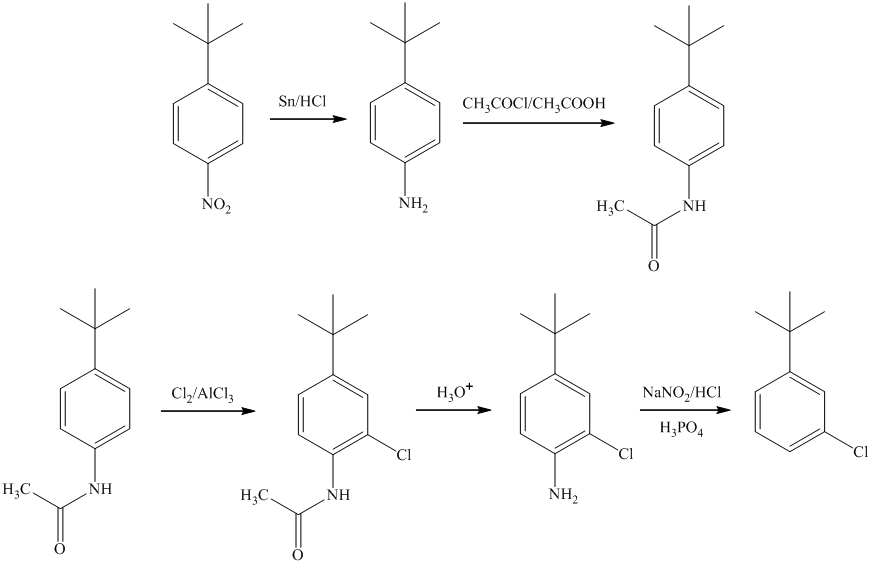
g)
In the synthesis of
In the final step, reduction of ketone occurs to yield the final product
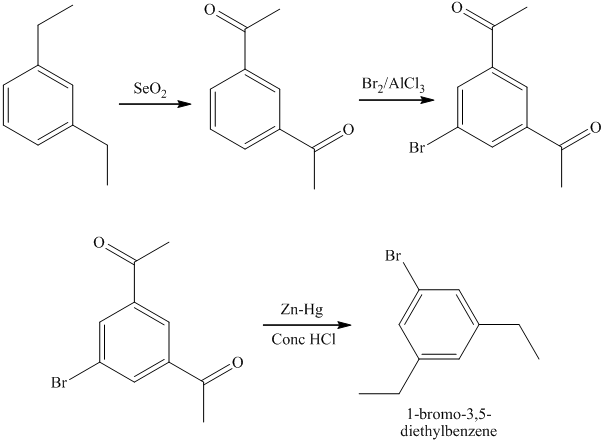
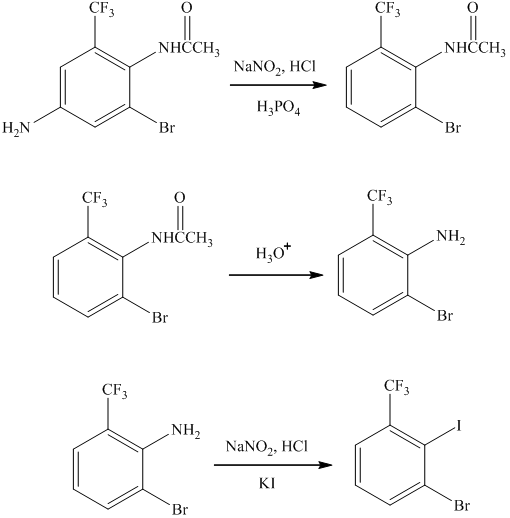
h)
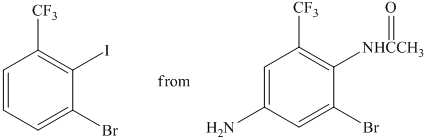
The preparation of
The synthesis of
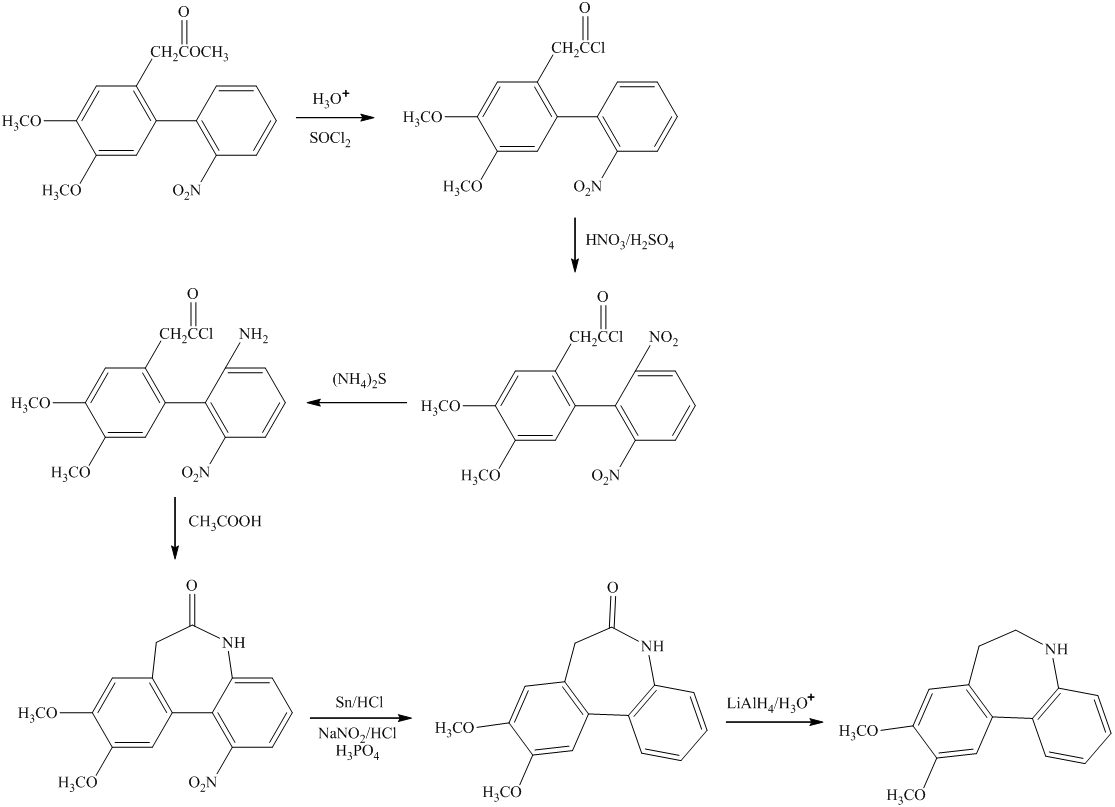
i)
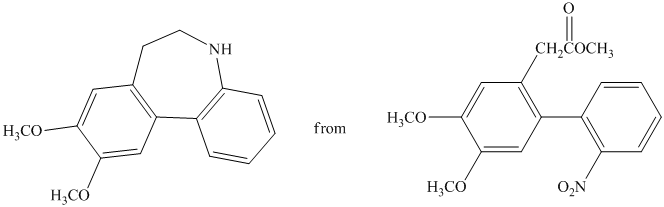
In this conversion, the formation of seven-membered ring present between two benzene rings occurs. Lithium aluminum hydride and sodium borohydride are strong reducing agents. The synthesis of the desired compound from its starting material is shown below.
Want to see more full solutions like this?
Chapter 22 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Cadaverine (1,5-diaminopentane) and putrescine (1,4-diaminobutane) are two compounds that are formed by bacterialaction and are responsible for the odor of rotting flesh. Drawtheir structures. Suggest a series of reactions to synthesize pu-trescine from 1,2-dibromoethane and any inorganic reagentsarrow_forwardSuggest a synthesis for the molecule below starting from benzene, alcohols of four carbons or fewer and any inorganic reagentsarrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)2,4,6-Trinitrotoluene (TNT)arrow_forward
- 4. Compound A has the formula C 8H 8. It reacts rapidly with KMnO 4 to give CO 2 and a carboxylic acid, B (C 7H 6O 2), but reacts with only 1 molar equivalent of H 2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H 2 are taken up and hydrocarbon C (C 8H 16) is produced. What are the structures of A, B, and C.arrow_forwardGive steucture of organic and inorganic products of the following sn2 reaction, and identify the nucleophile, substrate, and leaving group.arrow_forwardOutline the synthesis of racemic 3-methyl-3-heptanol, Et(Me)C(*)OHn-Bu, starting from alcohols of four carbons or fewer and any inorganic reagents or solvent needed.arrow_forward
- 3) Outline an acceptable step by step mechanism for the Nucleophilic aromatic substitution of ortho-fluoronitrobenzene with methylamine. Do not forget to include the formal charges of all non-hydrogen atoms that do not have a neutral charge.arrow_forwardCan you please check my work on the following ochem reaction scheme and let me know if it is correct or what is wrong... the question was: Consider 3,4-dimethylpiperidine being subjected to the following: Step 1: CH3I (excess); Step 2: NaOH, heat Step 3: CH3I (excess); Step 4: NaOH, heat Provide the bond line structures for the major organic product obtained in each step and discuss the regiochemistry for Step 2.arrow_forwardCompound A(C10H12O)gives off oxygen on treatment with sodium metal and also decolorizes Br2 in CCl4 to give organic compound B. Compound A on treatment with I2 in NaOH gives iodoform and salt C which after acidification gives a white solid D(C7H6O2). Using knowledge of organic chemistry identify structures A,B,C and Darrow_forward
- Wolff-Kishner reduction of compound W gave compound A. Treatment of A with m-chloroperbenzoic acid gave B which on reduction with LiAH4 gave C. Oxidation of compound C with chromic acid gave D (C9H14O). Suggest the structures for A, B, C, and D.arrow_forward(10pts) Compound A, C10H16, was found to be optically active. On catalytic reduction over a palladium catalyst, 2 equivalents of hydrogen were absorbed, yielding compound B, CioH2o. On ozonolysis of A, two fragments were obtained. One fragment was identified as acetic acid (CHCOOH). The other fragment, compound C, was an optically active carboxylic acid, C8H14O2. Write reactions, and draw the correct structures for A-C, explain your answer in detail.arrow_forward(i) Name, draw and describe the organic product of the reaction between 2-methylbut-1-ene and H2O in the presence of H2SO4 and provide a clear rationale as to why this is the major product of the reaction. (ii) The elimination reaction between 2-bromobutane and NaOCH2CH3 gives two organic products. Draw a mechanism for the reaction which produces the major organic elimination product and provide a rationale as to why that is the major product.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
