
Generl Chem Looself&mod Mst/et&stdy Crd Pkg, 11/e
1st Edition
ISBN: 9780134646534
Author: Petrucci
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 22, Problem 7E
Interpretation Introduction
(a)
Interpretation:
The probable geometric structure of
Concept introduction:
- Valence shell electron pair repulsion theory (VSEPR) predicts the geometry of the molecules depending on the number of bond pair of electrons and number of lone pair of electrons.
- Electron pair geometry includes both bonded atoms and lone pairs, while molecular geometry only considers the bonded atoms.
- The geometries corresponding to different steric number is shown − Steric number = number of bond pairs +number of lone pairs
| VSEPR GEOMETRIES | |||||
| Steric number | 0 lone pairs | 1 lone pair | 2 lone pairs | 3 lone pairs | 4 lone pairs |
| 2 | 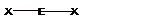 Linear |
||||
| 3 | Trigonal planar |
Bent |
|||
| 4 | Tetrahedral |
Trigonal pyramidal |
Bent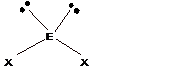 |
||
| 5 | TrigonalBipyramidal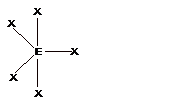 |
Seesaw |
T-shape |
Linear |
|
| 6 | Octahedral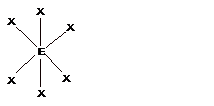 |
Square pyramidal |
Square planar |
T-shape |
Linear |
Interpretation Introduction
(b)
Interpretation:
The probable geometric structure of
Concept introduction:
- Valence shell electron pair repulsion theory (VSEPR) predicts the geometry of the molecules depending on the number of bond pair of electrons and number of lone pair of electrons.
- Electron pair geometry includes both bonded atoms and lone pairs, while molecular geometry only considers the bonded atoms.
- The geometries corresponding to different steric number is shown − Steric number = number of bond pairs +number of lone pairs
| VSEPR GEOMETRIES | |||||
| Steric number | 0 lone pairs | 1 lone pair | 2 lone pairs | 3 lone pairs | 4 lone pairs |
| 2 | 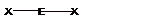 Linear |
||||
| 3 | Trigonal planar |
Bent |
|||
| 4 | Tetrahedral |
Trigonal pyramidal |
Bent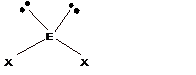 |
||
| 5 | TrigonalBipyramidal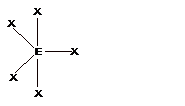 |
Seesaw |
T-shape |
Linear |
|
| 6 | Octahedral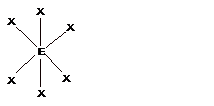 |
Square pyramidal |
Square planar |
T-shape |
Linear |
Interpretation Introduction
(c)
Interpretation:
The probable geometric structure of
Concept introduction:
- Valence shell electron pair repulsion theory (VSEPR) predicts the geometry of the molecules depending on the number of bond pair of electrons and number of lone pair of electrons.
- Electron pair geometry includes both bonded atoms and lone pairs, while molecular geometry only considers the bonded atoms.
- The geometries corresponding to different steric number is shown − Steric number = number of bond pairs +number of lone pairs
| VSEPR GEOMETRIES | |||||
| Steric number | 0 lone pairs | 1 lone pair | 2 lone pairs | 3 lone pairs | 4 lone pairs |
| 2 | 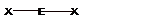 Linear |
||||
| 3 | Trigonal planar |
Bent |
|||
| 4 | Tetrahedral |
Trigonal pyramidal |
Bent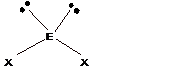 |
||
| 5 | Trigonal Bipyramidal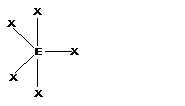 |
Seesaw |
T-shape |
Linear |
|
| 6 | Octahedral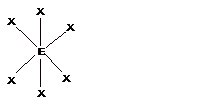 |
Square pyramidal |
Square planar |
T-shape |
Linear |
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Lithium oxide is an effective absorber of carbon dioxide and can be used to purify air in confined areas such as space vehicles. What volume of carbon dioxide can be absorbed by 1.00 kg of lithium oxide at 25°C and 1.00 atm? Li2O(aq) + CO2(g) → Li2CO3(s)
Write balanced chemical equations for the following reactions:(a) sodium oxide added to water(b) cesium carbonate added to an excess of an aqueous solution of HF(c) aluminum oxide added to an aqueous solution of HClO4(d) a solution of sodium carbonate added to solution of barium nitrate(e) titanium metal produced from the reaction of titanium tetrachloride with elemental sodium
What is the number of coulombs required to electrolyze Au⁺(aq) to produce 4.50 mol Au? (F = 96,500C/mol)
Chapter 22 Solutions
Generl Chem Looself&mod Mst/et&stdy Crd Pkg, 11/e
Ch. 22 - Give the formula of the stable fluride by Li, Be,...Ch. 22 - Fluorine is able to stabilize element’s in very...Ch. 22 - Prob. 3ECh. 22 - Prob. 4ECh. 22 - Prob. 5ECh. 22 - Prob. 6ECh. 22 - Prob. 7ECh. 22 - Use VSEPR theory to predict the probable geometric...Ch. 22 - Prob. 9ECh. 22 - Prob. 10E
Ch. 22 - Prob. 11ECh. 22 - Prob. 12ECh. 22 - Prob. 13ECh. 22 - Prob. 14ECh. 22 - Make a general prediction about which of the...Ch. 22 - The following properties of astatine have been...Ch. 22 - Prob. 17ECh. 22 - Prob. 18ECh. 22 - Prob. 19ECh. 22 - Prob. 20ECh. 22 - Prob. 21ECh. 22 - Prob. 22ECh. 22 - Prob. 23ECh. 22 - Prob. 24ECh. 22 - Each of the following compounds decomposes to...Ch. 22 - Ozone is a power oxidizing agent. Using ozone as...Ch. 22 - Prob. 27ECh. 22 - Prob. 28ECh. 22 - Prob. 29ECh. 22 - Prob. 30ECh. 22 - Prob. 31ECh. 22 - Prob. 32ECh. 22 - Prob. 33ECh. 22 - In water, O2(aq) is a strong base. If 100.0 mg of...Ch. 22 - The conversion of O2(g) to O2(g) can be...Ch. 22 - Prob. 36ECh. 22 - Prob. 37ECh. 22 - Prob. 38ECh. 22 - Prob. 39ECh. 22 - Prob. 40ECh. 22 - Prob. 41ECh. 22 - Joseph Priestley, e British chemist, was credited...Ch. 22 - Give an appropriate name to each of theb following...Ch. 22 - Prob. 44ECh. 22 - Give a specific example of a chemical equation...Ch. 22 - Prob. 46ECh. 22 - Prob. 47ECh. 22 - Prob. 48ECh. 22 - Prob. 49ECh. 22 - Prob. 50ECh. 22 - Prob. 51ECh. 22 - Prob. 52ECh. 22 - Prob. 53ECh. 22 - Prob. 54ECh. 22 - Prob. 55ECh. 22 - Prob. 56ECh. 22 - Prob. 57ECh. 22 - Prob. 58ECh. 22 - Prob. 59ECh. 22 - One reaction that competes with reaction (22.41),...Ch. 22 - Prob. 61ECh. 22 - Prob. 62ECh. 22 - Draw plausible Lewis structures for a....Ch. 22 - Both nitramide and hyponitrous acid have the...Ch. 22 - Prob. 65ECh. 22 - Prob. 66ECh. 22 - Prob. 67ECh. 22 - Prob. 68ECh. 22 - Prob. 69ECh. 22 - Prob. 70ECh. 22 - Use data from Table 7.2 (page 273) to calculate...Ch. 22 - Prob. 72ECh. 22 - Prob. 73ECh. 22 - Prob. 74ECh. 22 - Prob. 75ECh. 22 - What volume of H2(g) at 25C and 752 mmHg is...Ch. 22 - Prob. 77ECh. 22 - How many grams of CaH2(s) are required to generate...Ch. 22 - Prob. 79ECh. 22 - On the basis of molecular orbital theory, would...Ch. 22 - Prob. 81IAECh. 22 - Prob. 82IAECh. 22 - Prob. 83IAECh. 22 - The photograph was taken after a few drops of a...Ch. 22 - Prob. 85IAECh. 22 - Prob. 86IAECh. 22 - Prob. 87IAECh. 22 - Despite the fact that it has the higher molecular...Ch. 22 - The text mentions that ammonium perchlorate is an...Ch. 22 - Prob. 90IAECh. 22 - Prob. 91IAECh. 22 - Prob. 92IAECh. 22 - Refer to Figure 11-25 to arrange the following...Ch. 22 - Prob. 94IAECh. 22 - Prob. 95IAECh. 22 - Estimate the percent dissociation of CI2(g) into...Ch. 22 - Prob. 97IAECh. 22 - The structure of N(SiH2)2 involves a planar...Ch. 22 - Prob. 99IAECh. 22 - Refer to the Integrative Example on page 1082....Ch. 22 - The bond energies of CIz and 159kJmol1 are 243 and...Ch. 22 - Prob. 102IAECh. 22 - Prob. 103IAECh. 22 - Prob. 104IAECh. 22 - Prob. 105IAECh. 22 - The heavier halogens (CI, Br, and I) form...Ch. 22 - Prob. 107IAECh. 22 - Chemists have successfully synthesized the ionic...Ch. 22 - Prob. 109IAECh. 22 - Various thermochemical cycles are being explored...Ch. 22 - The decomposition of aqueous hydrogen peroxide is...Ch. 22 - Both in this chapter and in Chapter 19, we have...Ch. 22 - Prob. 113FPCh. 22 - The so-called pyroanions, X2O7n+ , form a series...Ch. 22 - A description of bonding in XeF2 based on the...Ch. 22 - Prob. 116FPCh. 22 - Prob. 117SAECh. 22 - Prob. 118SAECh. 22 - Prob. 119SAECh. 22 - Which of the following can oxidize Br to Br2 in...Ch. 22 - Prob. 121SAECh. 22 - Prob. 122SAECh. 22 - Prob. 123SAECh. 22 - Prob. 124SAECh. 22 - Prob. 125SAECh. 22 - Prob. 126SAECh. 22 - Prob. 127SAECh. 22 - Give a practical laboratory method that you might...Ch. 22 - Prob. 129SAECh. 22 - Prob. 130SAECh. 22 - Prob. 131SAECh. 22 - Prob. 132SAECh. 22 - Prob. 133SAECh. 22 - Prob. 134SAECh. 22 - Prob. 135SAE
Knowledge Booster
Similar questions
- Define the bonding that exists in metals and how this model explains some of the unique physical properties of metals. What are metal alloys? Identify the two main types of alloys, and describe how their structures differ. Give several examples of each type of alloy.arrow_forwardWhat is the oxidation state of the noble gas in each of the following? You may wish to review the chapter on chemical bonding and molecular geometry. (a) XeO2F2. (b) KrF2. (c) XeF3+. (d) XeO64-. (e) XeO3arrow_forwardFor each of the following draw the Lewis structure, predict the ONO bond angle, and give the hybridization of the nitrogen. You may wish to review the chapters on chemical bonding and advanced theories of covalent bonding for relevant examples. (a) NO2. (b) NO2-. (c) NO2+arrow_forward
- Describe the hybridization of silicon and the molecular structure of the following molecules and ions: (a) (CH3)3SiH. (b) SIO44-. (c) Si2H6. (d) Si(OH)4. (e) SiF62-arrow_forward(i) Interhalogen compounds are more reactive than halogens exceptF2. Why?(ii) Give one important use of ClF3.arrow_forwardWrite the Lewis structure for each of the following species,describe its geometry, and indicate the oxidation state ofthe nitrogen: (a) HNO2, (b) N3- , (c) N2H5+, (d) NO3- .arrow_forward
- a. Hydrogen Sulphide (H2S) is a by-product often associated with 0il & Gas exploration and the refining industries. Explain the dangers of this substance and what Personal Protection Equipment (PPE) should be used, to aid survival when coming into contact with this substance.arrow_forwardA seawater sample contains 2.5% NaClNaCl (MW=58.44 g/mol) by mass. Calculate the normal boiling point of seawater. (KbKb=0.512°C/m)arrow_forwardGive the hybridization and oxidation state for sulfur in SO2, in SO3, and in H2SO4.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning