
Interpretation: Using a Gabriel synthesis, a given primary amine compound has to be synthesized.
Concept Introduction:
The general formula for primary amine is –NH2. There are several methods available to prepare primary
Formation of potassium phthalimide (deprotonation)
Potassium phthalimide in alkaline KOH acts as the reagent which has negatively charged phthalimide. It is formed by the reaction between phthalimide and potassium hydroxide.
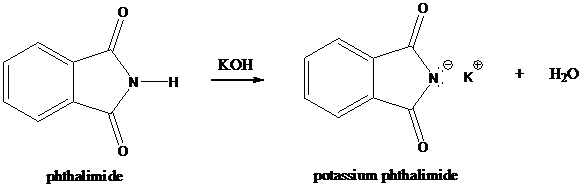
Formation of R−N bond by SN2 nucleophilic substitution
The negative charged nitrogen atom in phthalimide can easily attract the positive side of R−X. In primary
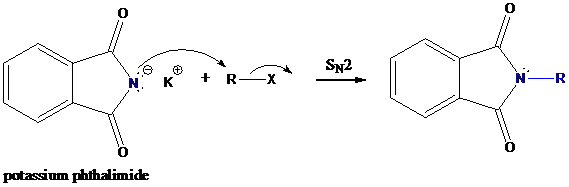
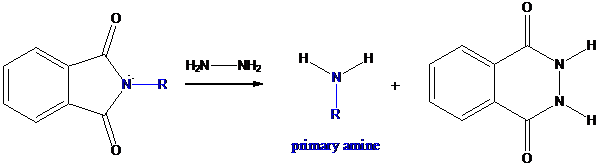
Formation of primary amine by hydrolysis
The resultant product further goes for hydrolysis using hydrazine as the reagent. This reaction also follows nucleophilic substitution reaction. Finally, primary amine is formed with a side product of hydrazine derivative.
To find: Using a Gabriel synthesis, prepare the given primary amine compound
Identify the correct alkyl halide involved in the formation of the given compound
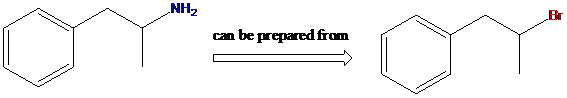
Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
KLEIN'S ORGANIC CHEMISTRY
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





