
Concept explainers
(a)
Interpretation: To indicate whether oxaloacetate undergoes (1) oxidation but not reduction, (2) reduction but not oxidation, (3) both oxidation and reduction or (4) neither oxidation nor reduction in the common
Concept introduction: Common metabolic pathway is the total sum of metabolic reactions that occur in stage third and fourth of the biochemical process or it is defined as the total sum of reactions that occur in the citric acid cycle and electron transport chain and oxidative phosphorylation.
These stages are included in the common metabolic pathway because the reactions in these stages are the same for different kinds of food.
The citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
(a)
Answer to Problem 23.103EP
Oxaloacetate undergoes neither oxidation nor reduction in the common metabolic pathway.
Explanation of Solution
Oxaloacetate undergoes condensation reaction in the first step of the citric acid cycle.
The first step involves the condensation reaction of oxaloacetate and
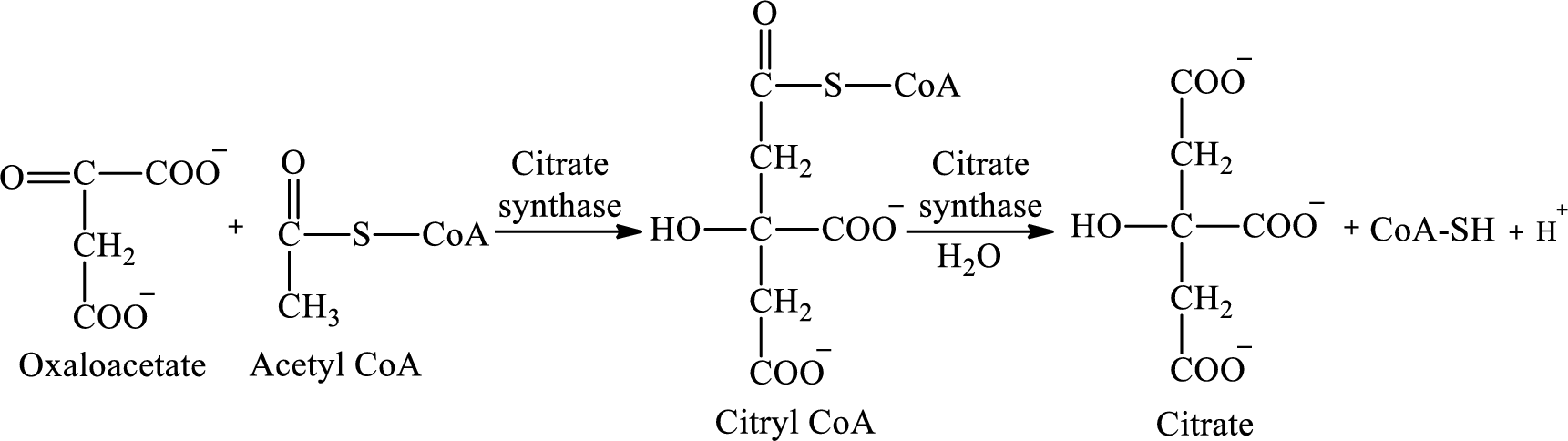
(b)
Interpretation: To indicate whether
Concept introduction: Common metabolic pathway is the total sum of metabolic reactions that occur in stage third and fourth of the biochemical process or it is defined as the total sum of reactions that occur in the citric acid cycle and electron transport chain and oxidative phosphorylation.
These stages are included in the common metabolic pathway because the reactions in these stages are the same for different kinds of food.
The citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
(b)
Answer to Problem 23.103EP
Explanation of Solution
Nicotinamide adenine dinucleotide exists in two forms: oxidized form
The reaction of step 3 is:
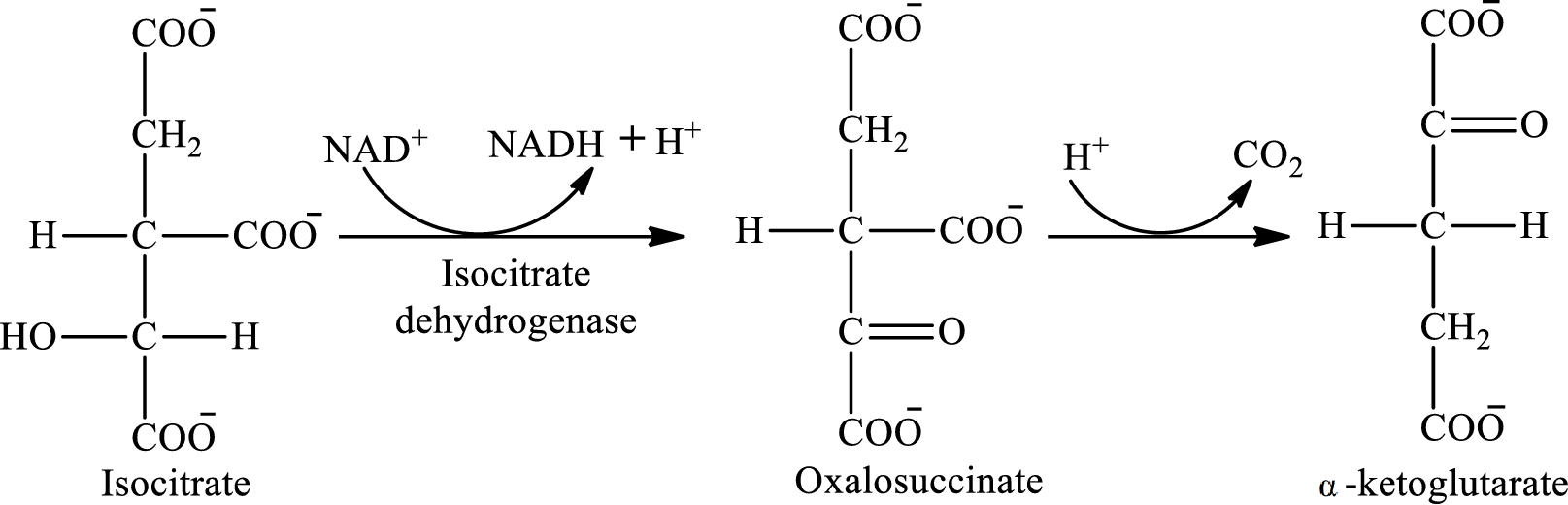
The reaction of step 4 is:
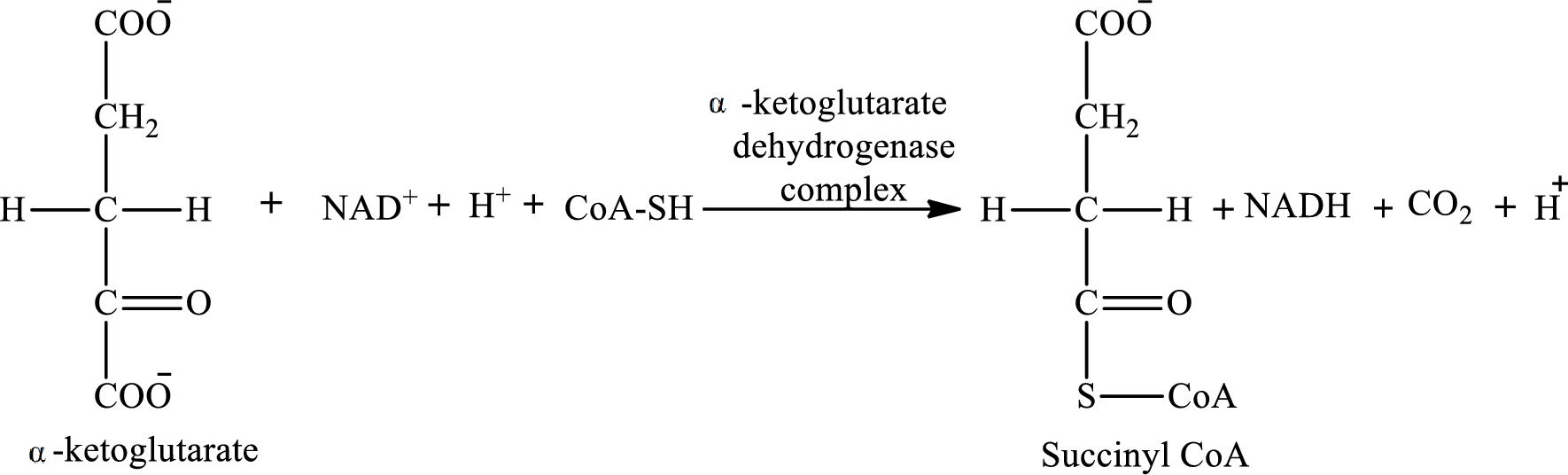
The reaction of step 8 is:
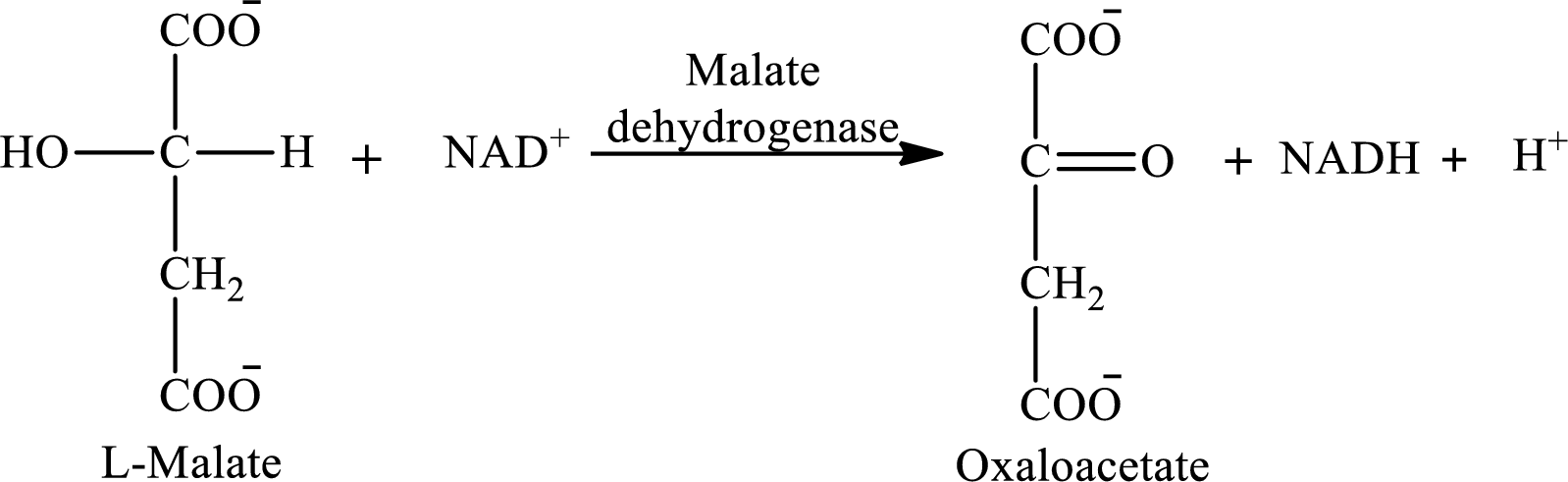
(c)
Interpretation: To indicate whether FADH2 undergoes (1) oxidation but not reduction, (2) reduction but not oxidation, (3) both oxidation and reduction, or (4) neither oxidation nor reduction in the common metabolic pathway.
Concept introduction: Common metabolic pathway is the total sum of metabolic reactions that occur in stage third and fourth of the biochemical process or it is defined as the total sum of reactions that occur in the citric acid cycle and electron transport chain and oxidative phosphorylation.
These stages are included in the common metabolic pathway because the reactions in these stages are the same for different kinds of food.
The citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
(c)
Answer to Problem 23.103EP
FADH2 undergoes oxidation in the common metabolic pathway.
Explanation of Solution
FADH2is the reduced form of flavin adenine dinucleotide. The main function of flavin adenine dinucleotide is to act as an oxidizing agent and used by the cell in oxidation reactions like oxidation of fatty acid. The reaction of the oxidation of FADH2 is:
FADH2undergoes oxidation in complex II of the electron transport chain.
Complex II consists of four subunits in its structure. This complex interacts initially with the electrons that are coming after the reduction of FADH2. FADH2 is oxidized to form FAD in this reaction. The diagrammatic representation of electron transfer in complex II in the electron transfer chain is:
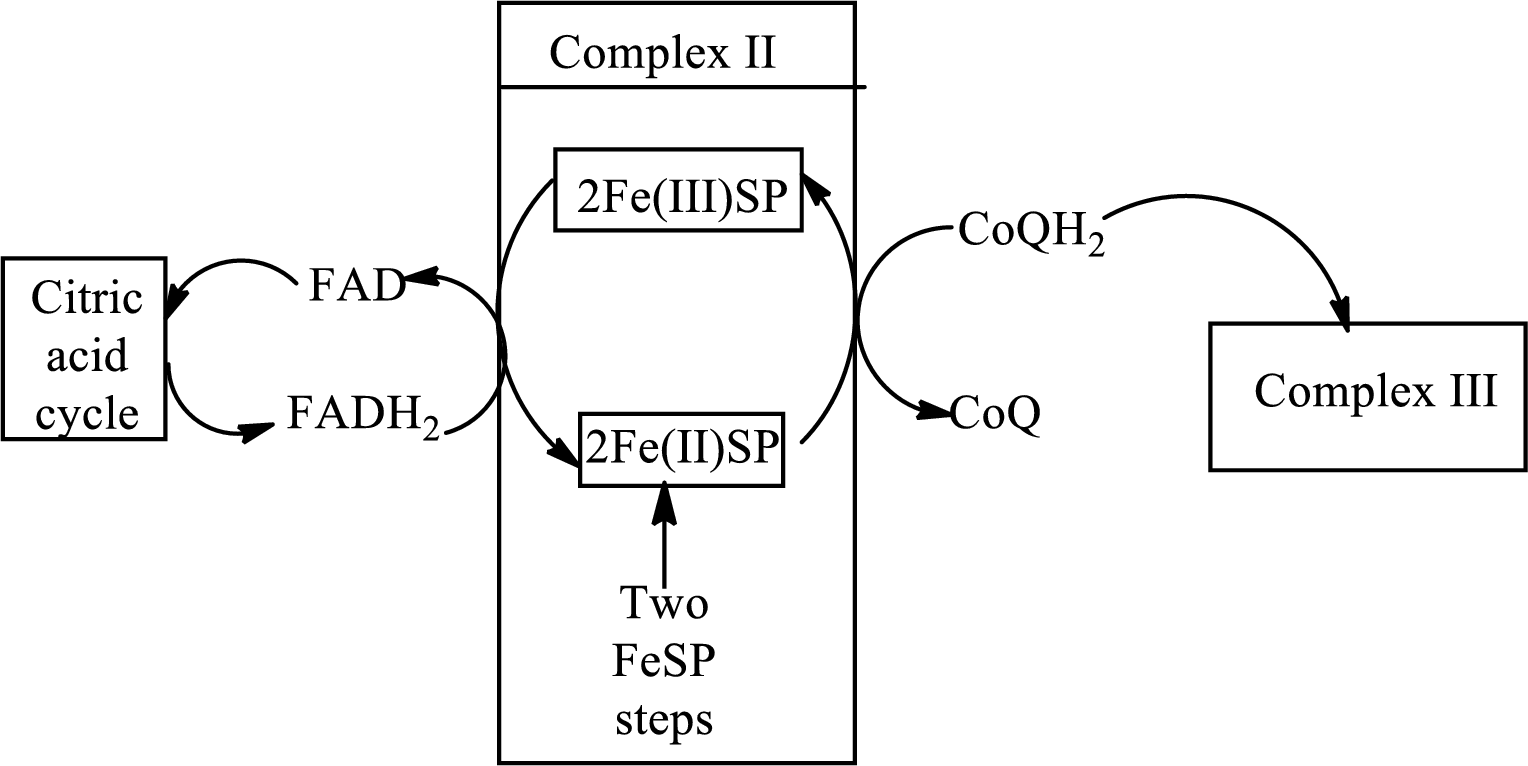
(d)
Interpretation: To indicate whether
Concept introduction: Common metabolic pathway is the total sum of metabolic reactions that occur in stage third and fourth of the biochemical process or it is defined as the total sum of reactions that occur in the citric acid cycle and electron transport chain and oxidative phosphorylation.
These stages are included in the common metabolic pathway because the reactions in these stages are the same for different kinds of food.
The citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
(d)
Answer to Problem 23.103EP
Explanation of Solution
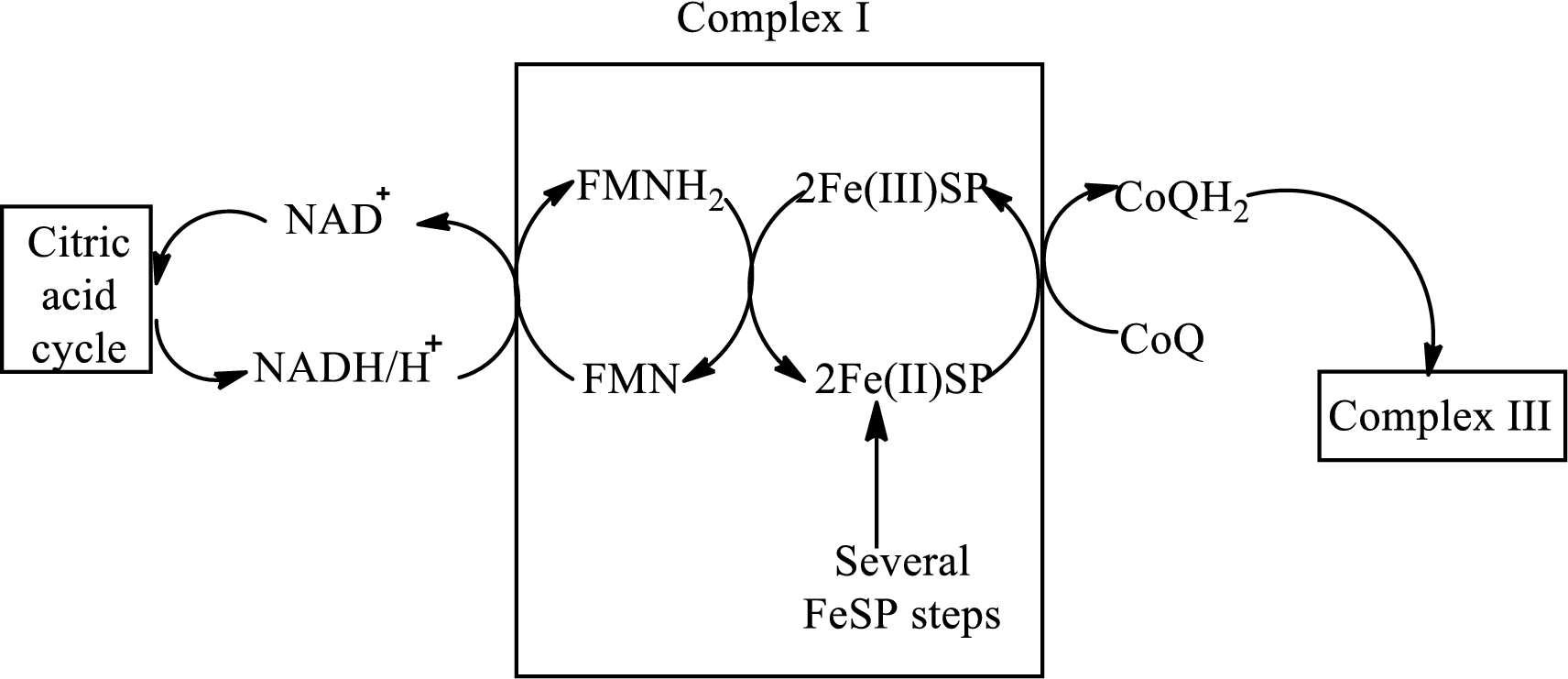
Complex I consists of more than 40 structural subunits. Its structure has B-vitamin-containing flavin mononucleotide
The diagrammatic representation of electron transfer in complex I in the electron transfer chain is as follows:
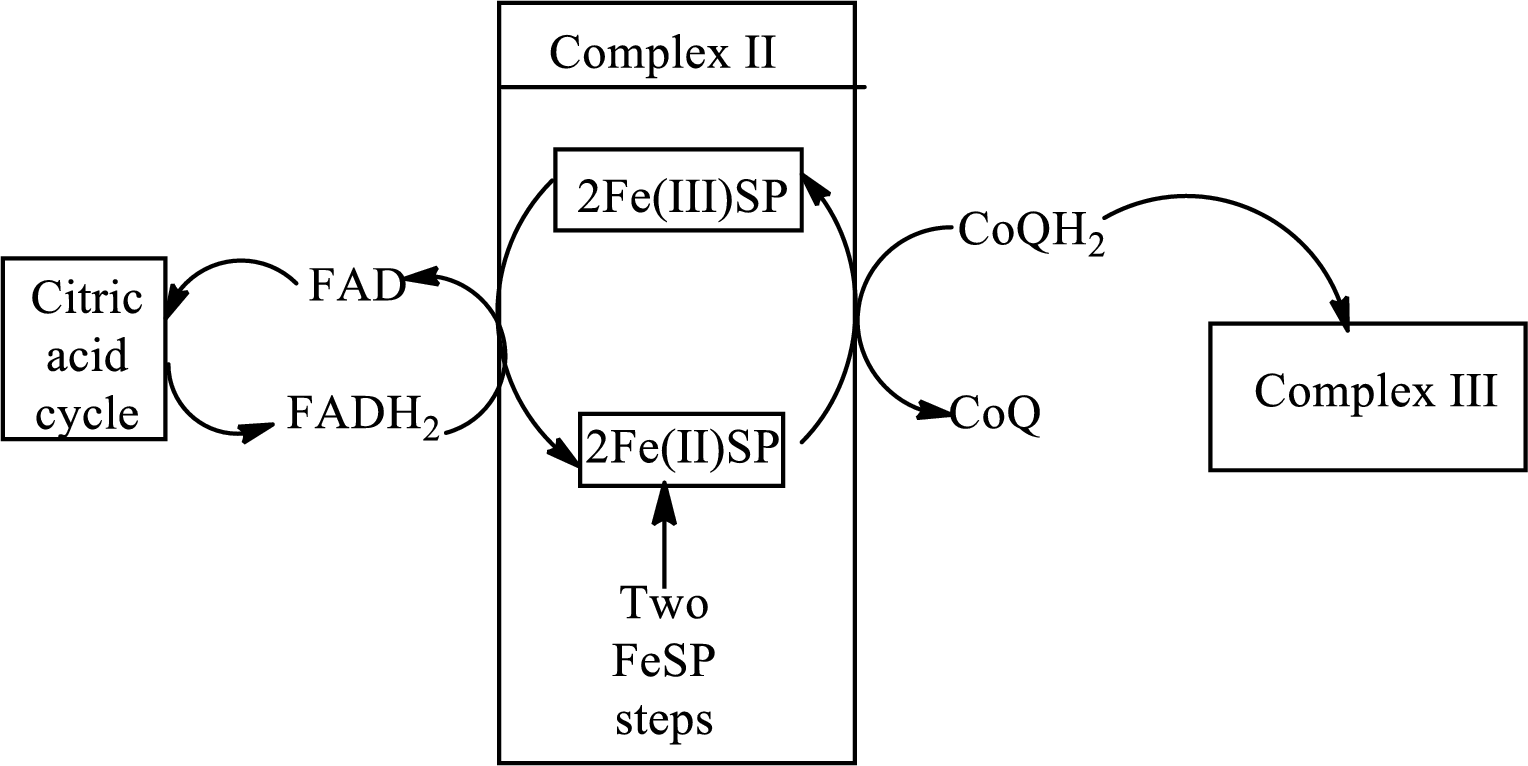
Complex II consists of four subunits in its structure. This complex interacts initially with the electrons that are coming after the reduction of FADH2. FADH2 produced in the citric acid cycle transfers the electron to the complex II. The diagrammatic representation of electron transfer in complex II in the electron transfer chain is:
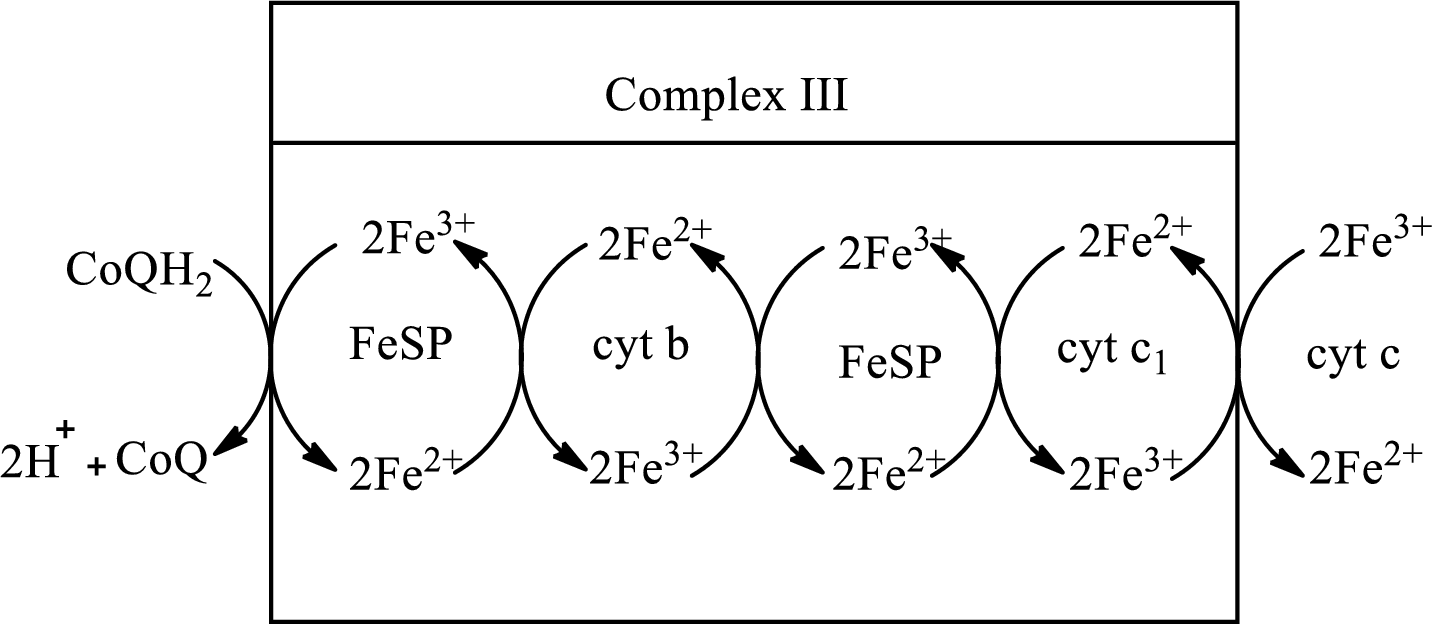
Complex III consists of 11 structural subunits. Its structural subunits contain iron-sulfur proteins and various cytochromes.
Want to see more full solutions like this?
Chapter 23 Solutions
General, Organic, and Biological Chemistry
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning




