
Laboratory Manual for Chemistry (7th Edition)
7th Edition
ISBN: 9780133886627
Author: John E. McMurry, Robert C. Fay, Jill Kirsten Robinson, Stephanie Dillon, Sandra Rogers
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 23, Problem 23.114SP
Interpretation Introduction
Interpretation:
The hybridization of each N, O and, S atom in vitamin B7 should be determined.
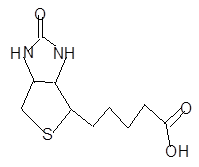
Concept introduction:
Hybridization is a term of mixing of atomic orbital having same energies to produce new orbital and these atomic orbitals are known as hybrid orbitals. In hybridization only, sigma bond participates not pi.
If two atomic orbitals combine then hybridization is sp, in case of three atomic orbitals the hybridization is sp2and in case of four atomic orbitals the hybridization is sp3.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Laboratory Manual for Chemistry (7th Edition)
Ch. 23 - Prob. 23.1PCh. 23 - Prob. 23.2ACh. 23 - Prob. 23.3PCh. 23 - Prob. 23.4ACh. 23 - Prob. 23.5PCh. 23 - Prob. 23.6PCh. 23 - Prob. 23.7PCh. 23 - Prob. 23.8ACh. 23 - Prob. 23.9PCh. 23 - Prob. 23.10A
Ch. 23 - Prob. 23.11PCh. 23 - Prob. 23.12PCh. 23 - Prob. 23.13ACh. 23 - Prob. 23.14PCh. 23 - Prob. 23.15ACh. 23 - Prob. 23.16PCh. 23 - Prob. 23.17ACh. 23 - Prob. 23.18PCh. 23 - Prob. 23.19PCh. 23 - Prob. 23.20ACh. 23 - Prob. 23.21PCh. 23 - Prob. 23.22ACh. 23 - Prob. 23.23PCh. 23 - Prob. 23.24ACh. 23 - Prob. 23.25PCh. 23 - Prob. 23.26PCh. 23 - Prob. 23.27ACh. 23 - Prob. 23.28PCh. 23 - Prob. 23.29ACh. 23 - Prob. 23.30PCh. 23 - Prob. 23.31ACh. 23 - Prob. 23.32PCh. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - Prob. 23.35CPCh. 23 - Prob. 23.36CPCh. 23 - Prob. 23.37CPCh. 23 - Prob. 23.38CPCh. 23 - Prob. 23.39CPCh. 23 - Prob. 23.40CPCh. 23 - Prob. 23.41CPCh. 23 - Prob. 23.42SPCh. 23 - Prob. 23.43SPCh. 23 - Prob. 23.44SPCh. 23 - Prob. 23.45SPCh. 23 - Prob. 23.46SPCh. 23 - Prob. 23.47SPCh. 23 - Prob. 23.48SPCh. 23 - Prob. 23.49SPCh. 23 - Prob. 23.50SPCh. 23 - Prob. 23.51SPCh. 23 - Prob. 23.52SPCh. 23 - Prob. 23.53SPCh. 23 - Prob. 23.54SPCh. 23 - Prob. 23.55SPCh. 23 - Prob. 23.56SPCh. 23 - Prob. 23.57SPCh. 23 - Prob. 23.58SPCh. 23 - Prob. 23.59SPCh. 23 - Prob. 23.60SPCh. 23 - Prob. 23.61SPCh. 23 - Prob. 23.62SPCh. 23 - Prob. 23.63SPCh. 23 - Prob. 23.64SPCh. 23 - Prob. 23.65SPCh. 23 - Prob. 23.66SPCh. 23 - Prob. 23.67SPCh. 23 - Prob. 23.68SPCh. 23 - Prob. 23.69SPCh. 23 - Prob. 23.70SPCh. 23 - Prob. 23.71SPCh. 23 - Prob. 23.72SPCh. 23 - Prob. 23.73SPCh. 23 - Prob. 23.74SPCh. 23 - Prob. 23.75SPCh. 23 - Prob. 23.76SPCh. 23 - Prob. 23.77SPCh. 23 - Prob. 23.78SPCh. 23 - Prob. 23.79SPCh. 23 - Prob. 23.80SPCh. 23 - Prob. 23.81SPCh. 23 - Prob. 23.82SPCh. 23 - Prob. 23.83SPCh. 23 - Prob. 23.84SPCh. 23 - Prob. 23.85SPCh. 23 - Prob. 23.86SPCh. 23 - Prob. 23.87SPCh. 23 - Prob. 23.88SPCh. 23 - Prob. 23.89SPCh. 23 - Prob. 23.90SPCh. 23 - Prob. 23.91SPCh. 23 - Prob. 23.92SPCh. 23 - Draw the structure of a fatty acid with a lipid...Ch. 23 - Prob. 23.94SPCh. 23 - Prob. 23.95SPCh. 23 - Prob. 23.96SPCh. 23 - Prob. 23.97SPCh. 23 - Prob. 23.98SPCh. 23 - Prob. 23.99SPCh. 23 - Prob. 23.100SPCh. 23 - Prob. 23.101SPCh. 23 - Prob. 23.102SPCh. 23 - Prob. 23.103SPCh. 23 - Prob. 23.104SPCh. 23 - Prob. 23.105SPCh. 23 - Prob. 23.106SPCh. 23 - Prob. 23.107SPCh. 23 - Prob. 23.108SPCh. 23 - Prob. 23.109SPCh. 23 - Prob. 23.110SPCh. 23 - Prob. 23.111SPCh. 23 - Prob. 23.112SPCh. 23 - In the following molecules, indicate which atoms...Ch. 23 - Prob. 23.114SPCh. 23 - Prob. 23.115SPCh. 23 - Prob. 23.116SPCh. 23 - Prob. 23.117SPCh. 23 - Prob. 23.118SPCh. 23 - Prob. 23.119SPCh. 23 - Prob. 23.120SPCh. 23 - Prob. 23.121SPCh. 23 - Prob. 23.122SPCh. 23 - Prob. 23.123SPCh. 23 - Prob. 23.124SPCh. 23 - Prob. 23.125SPCh. 23 - Prob. 23.126SPCh. 23 - Prob. 23.127SPCh. 23 - Prob. 23.128SPCh. 23 - Prob. 23.129SPCh. 23 - Prob. 23.130SPCh. 23 - Prob. 23.131SPCh. 23 - Prob. 23.132SPCh. 23 - Prob. 23.133SPCh. 23 - Prob. 23.134SPCh. 23 - Prob. 23.135SPCh. 23 - Prob. 23.136CPCh. 23 - Prob. 23.137CPCh. 23 - Prob. 23.138CPCh. 23 - Prob. 23.139CPCh. 23 - Prob. 23.140CPCh. 23 - Prob. 23.141CPCh. 23 - Prob. 23.142CPCh. 23 - Prob. 23.143CPCh. 23 - Prob. 23.144CPCh. 23 - Prob. 23.145CPCh. 23 - Prob. 23.146CPCh. 23 - Prob. 23.147CPCh. 23 - Prob. 23.148CPCh. 23 - Prob. 23.149CPCh. 23 - Prob. 23.150CPCh. 23 - Prob. 23.151CPCh. 23 - Prob. 23.152MPCh. 23 - Prob. 23.153MPCh. 23 - Prob. 23.154MPCh. 23 - Prob. 23.155MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY