
(a)
Interpretation:
How to synthesize the given trisubstituted benzene from the disubstituted benzene is to be shown.
Concept introduction:
If multiple substituents are attached to a phenyl ring, their activating/deactivating qualities are additive, and the regiochemistry tends to be governed by the most activating of those substituents.
The alkyl group is ortho/para director whereas
Answer to Problem 23.55P
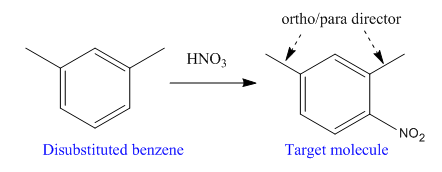
The disubstituted benzene used to synthesize the given trisubstituted benzene is
Explanation of Solution
The given trisubstituted target molecule is
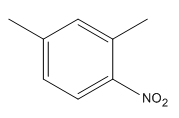
In the above molecule,
This reaction can be carried out using addition of nitric acid
Therefore, the product could be produced by nitrating

The disubstituted benzene used to synthesize the given trisubstituted benzene is shown on the basis of the directing nature of the substituents present at the benzene ring on the target molecule (given trisubstituted benzene).
(b)
Interpretation:
How to synthesize the given trisubstituted benzene from the disubstituted benzene is to be shown.
Concept introduction:
If multiple substituents are attached to a phenyl ring, their activating/deactivating qualities are additive, and the regiochemistry tends to be governed by the most activating of those substituents.
Answer to Problem 23.55P
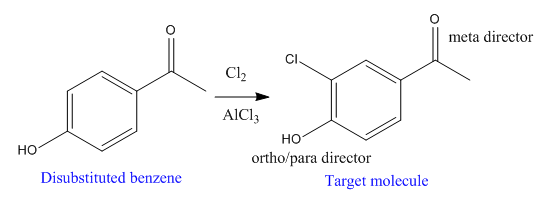
The disubstituted benzene used to synthesize the given trisubstituted benzene is
Explanation of Solution
The given trisubstituted target molecule is
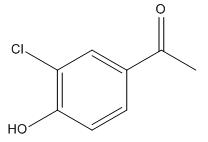
In the above molecule,
Thus, the product could be produced by chlorinating the disubstituted benzene using

The disubstituted benzene used to synthesize the given trisubstituted benzene is shown on the basis of the directing nature of the substituents present at the benzene ring on the target molecule (given trisubstituted benzene).
(c)
Interpretation:
How to synthesize the given trisubstituted benzene from the disubstituted benzene is to be shown.
Concept introduction:
If multiple substituents are attached to a phenyl ring, their activating/deactivating qualities are additive, and the regiochemistry tends to be governed by the most activating of those substituents.
Answer to Problem 23.55P
The disubstituted benzene used to synthesize the given trisubstituted benzene is
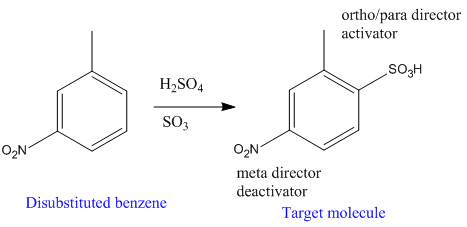
Explanation of Solution
The given trisubstituted target molecule is
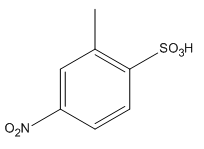
In the above molecule,
Therefore, the product could be produced by sulfonating the disubstituted benzene using fuming

The disubstituted benzene used to synthesize the given trisubstituted benzene is shown on the basis of the directing nature of the substituents present at the benzene ring on the target molecule (given trisubstituted benzene).
(d)
Interpretation:
How to synthesize the given trisubstituted benzene from the disubstituted benzene is to be shown.
Concept introduction:
If multiple substituents are attached to a phenyl ring, their activating/deactivating qualities are additive, and the regiochemistry tends to be governed by the most activating of those substituents.
Answer to Problem 23.55P
The disubstituted benzene used to synthesize the given trisubstituted benzene is
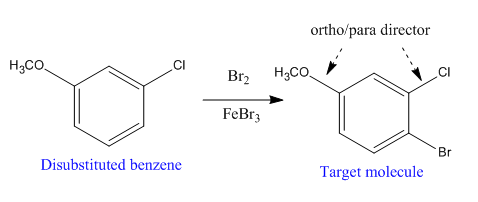
Explanation of Solution
The given trisubstituted target molecule is
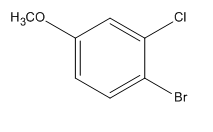
Here, both
Therefore, the target molecule could be produced by brominating the disubstituted benzene using

The disubstituted benzene used to synthesize the given trisubstituted benzene is shown on the basis of the directing nature of the substituents present at the benzene ring on the target molecule (given trisubstituted benzene).
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY:PRINCIPLES...(CL)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





