
Concept explainers
(a)
Interpretation:
For the given complex the orbital splitting diagram has to be drawn using spectrochemial series.
Concept introduction:
The element in the periodic table and count its position in the respective transition series. These elements are in Periods 5 and 6, so the general configuration is
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
Splitting of five d-orbitals in an octahedral crystals field is as follows:
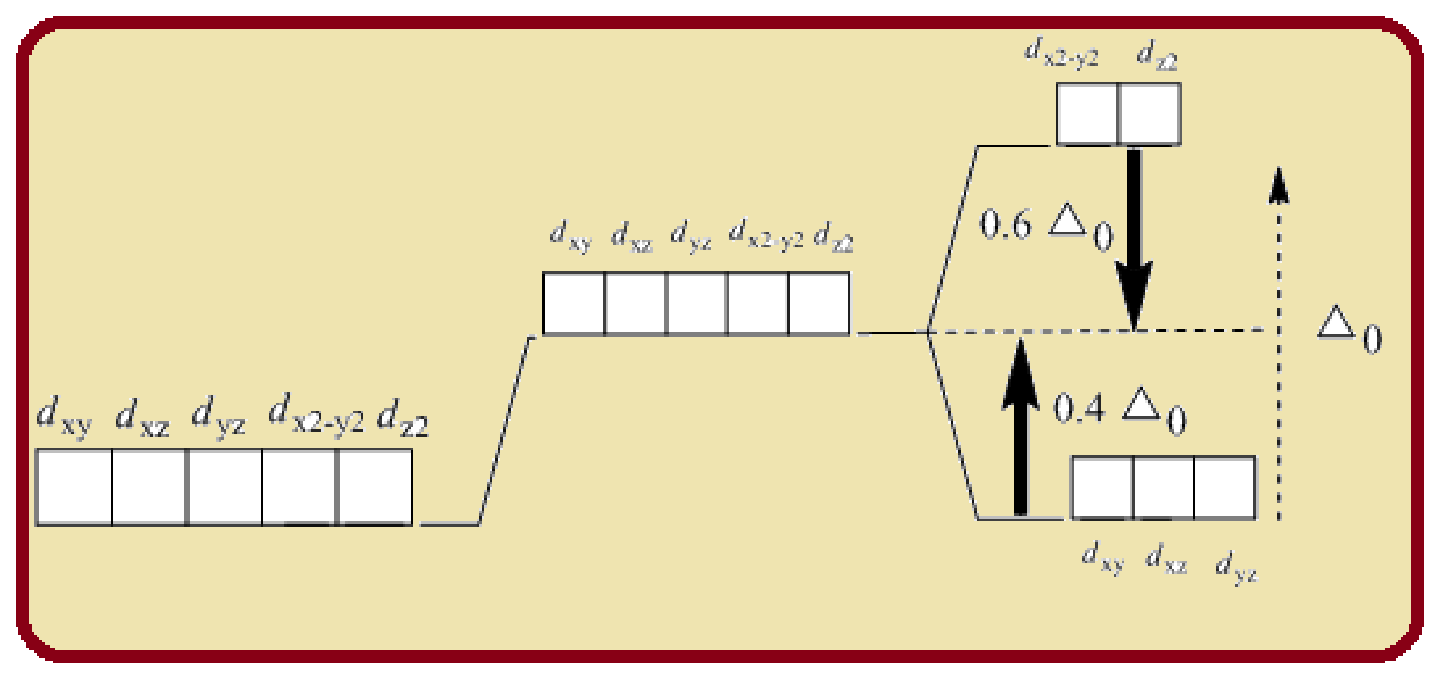
Figure 1
(a)
Explanation of Solution
Given,
Electron configuration of
Charge on
Electron configuration of
Six ligands indicate an octahedral arrangement. Using Hund’s rule, fill the lower energy
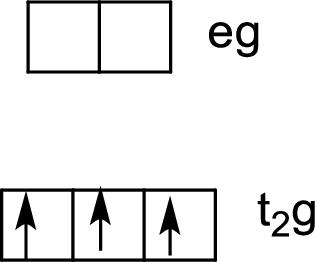
(b)
Interpretation:
For the given complex the orbital splitting diagram has to be drawn using spectrochemial series.
Concept introduction:
The element in the periodic table and count its position in the respective transition series. These elements are in Periods 5 and 6, so the general configuration is
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
Splitting of five d-orbitals in an octahedral crystals field is as follows:
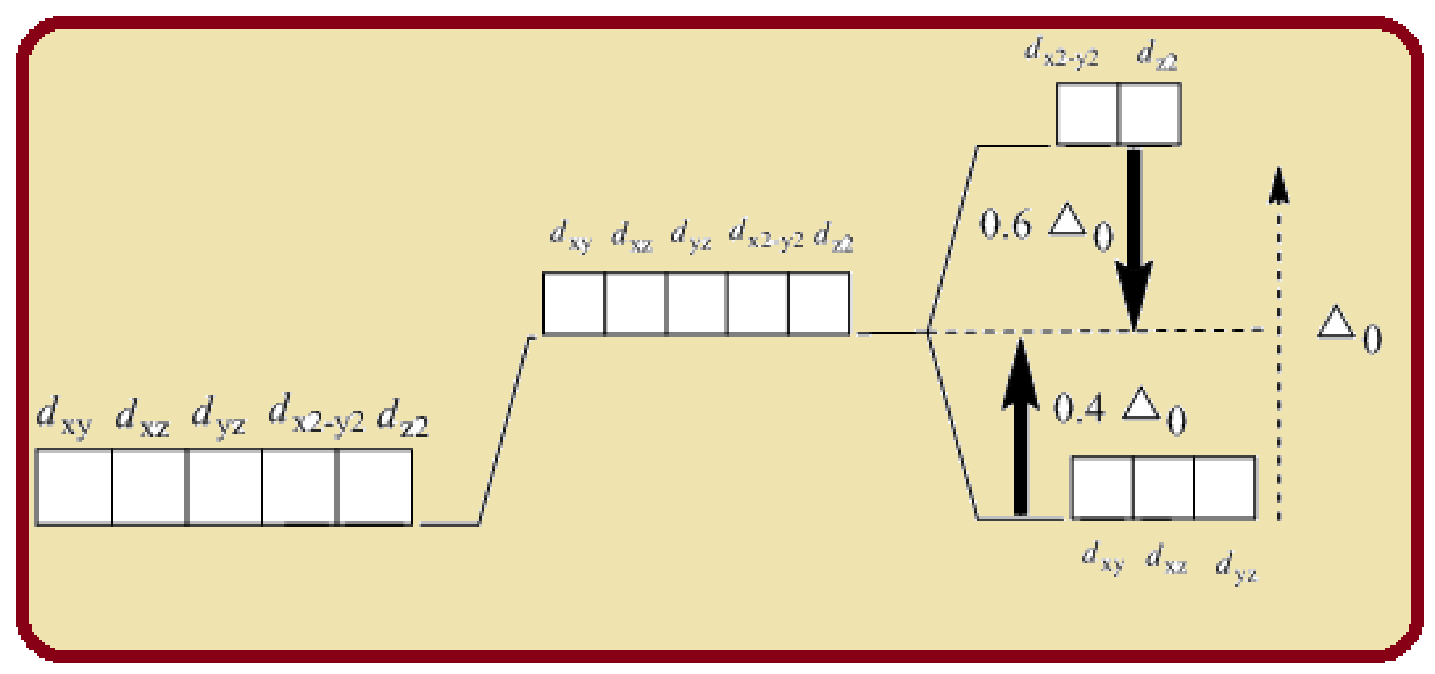
Figure 1
(b)
Explanation of Solution
Given,
Electron configuration of
Charge on
Electron configuration of
Four ligands and a
(c)
Interpretation:
For the given complex the orbital splitting diagram has to be drawn using spectrochemial series.
Concept introduction:
The element in the periodic table and count its position in the respective transition series. These elements are in Periods 5 and 6, so the general configuration is
Spectrochemical series: The list of ligands arranged in an ascending order of
Crystal field splitting: The energy gap between the splitting of d-orbitals of the metal ion in presence of ligands is known as the crystal field splitting
Splitting of five d-orbitals in an octahedral crystals field is as follows:
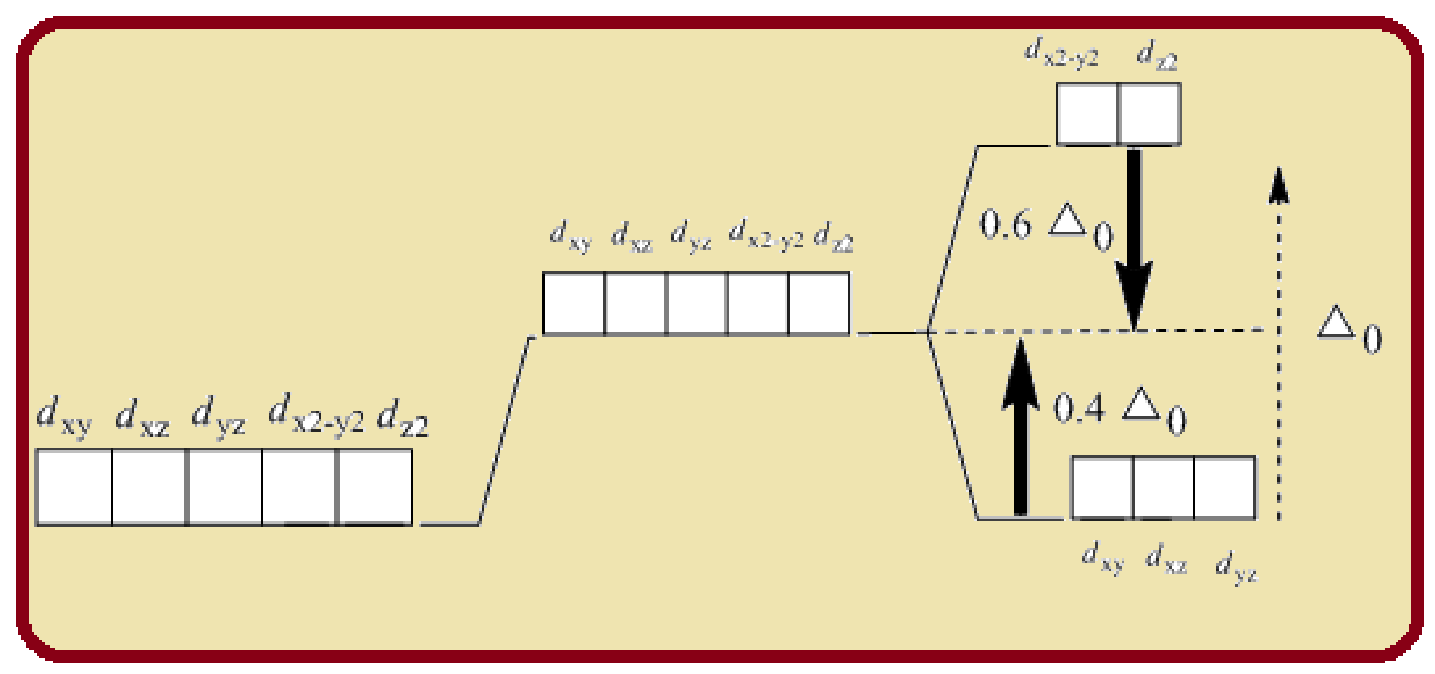
Figure 1
(c)
Explanation of Solution
Electron configuration of
Charge on
Electron configuration of
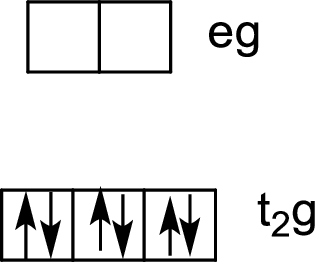
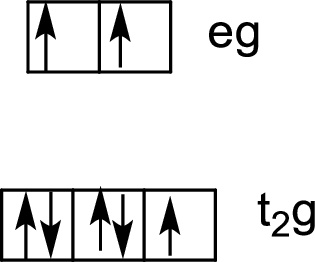
Six ligands indicate an octahedral arrangement. Use Hund’s rule to fill the orbitals.
Water – is a weak-field ligand, so the splitting energy, Δ, is not large enough to overcome the resistance to electron pairing. The electrons remain unpaired, and the complex is called high-spin.
Want to see more full solutions like this?
Chapter 23 Solutions
EBK CHEMISTRY: THE MOLECULAR NATURE OF
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





