
Concept explainers
(a)
Interpretation:
The hybridization and bond angles of carbon is bonded via four single bonds to adjacent atoms should be determined.
Concept introduction:
Carbon
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Example – Ethane.
Example –
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(a)
Answer to Problem 2PS
The carbon is
Explanation of Solution
Carbon is bonded via four single bonds to adjacent atoms. Carbon is bonded with four single four hydrogen atoms.
The Lewis structure as shown below.

Let’s write the carbon electronic configuration:
Here, the carbon is
(b)
Interpretation:
The hybridization and bond angle of carbon which is bonded via two single bonds and one pi bond should be determined.
Concept introduction:
Carbon atomic number 6. Electronic configuration -
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Example – Ethane.
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(b)
Answer to Problem 2PS
The carbon is
Explanation of Solution
Carbon is bonded via two single bonds and one pi bond.
Side overlapping of the two adjacent carbon atoms of orbitals form pi-bond.
The Lewis structure as shown below.
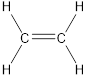
Let’s find the hybridization:
Here, the carbon is
(c)
Interpretation:
The hybridization and bond angles of carbon is bonded via one single bond and one triple bond should be determined.
Concept introduction:
Carbon atomic number 6. Electronic configuration -
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(c)
Answer to Problem 2PS
The carbon is
Explanation of Solution
Carbon is bonded via one single bond and one triple bond. Three pairs of electron are shared by two adjacent carbon atoms.
The Lewis structure as shown below.

Let’s find the Hybridization:
Here, the carbon is
(d)
Interpretation:
The hybridization and bond angles of carbon which are bonded via two double bonds should be determined.
Concept introduction:
Carbon atomic number 6. Electronic configuration -
Carbon has tetra valency. It is bonded with four bonds to adjacent atoms or molecules.
Single covalent bond - one pair of each electrons are shared.
Double covalent bond – two pair of electrons are shared.
Triple covalent bond – Three pairs of electron are shared.
Hybridization: The phenomenon of formation new orbitals by the mixing of atomic orbital’s with equal energy.
Sp hybridization: Mixing of one –‘s’ orbital and one ‘p’ orbital. And form new hybrid orbital. Angle is
Example -
Example – Ethylene.
Example – Ethane.
Bond angle is the angle between two bonds of a molecule and it is determined based on the electron-domain geometry.
[Bond angles: tetrahedral =
(d)
Answer to Problem 2PS
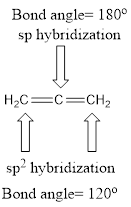
Explanation of Solution
Carbon is bonded via two double bonds. The three adjacent carbon atoms orbitals overlap to form two pi bonds.
The Lewis structure as shown below.
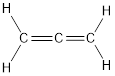
Let’s find the hybridization:
- (a) Hybridization of the terminal carbon atoms
Here, the carbon is
- (b) Hybridization of the central carbon atom
Here, the carbon is
Therefore,
The hybridization and bond angle is

Want to see more full solutions like this?
Chapter 23 Solutions
CHEMISTRY+CHEM.REACT. (LOOSELEAF)
- What type of hybridized orbital is present on carbon atoms bonded by a triple bond? How many of these hybrid orbitals are on each carbon atom?arrow_forwardDraw the structure and give the molecular formula for a compound(a) 1-ethyl-3-methylcycloheptanearrow_forwardDraw the cis and trans of 2-methyl-3-hexene and condensed structure and skeletal structure of it.arrow_forward
- Draw and name all the constitutional isomers of trimethyl-benzenearrow_forwardIndicate whether each molecule is an aliphatic or an aromatic hydrocarbon. If it is aliphatic, identify the molecule as an alkane, an alkene, or an alkyne.arrow_forwardWhat is the name of the compound if it is one of the first 10 noncyclic, unbranched alkanes and what is the mass% of hydrogen in the compound?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





