
Concept explainers
(a)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
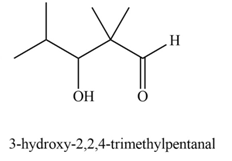
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
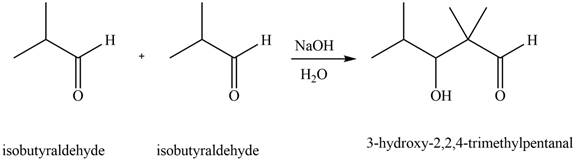
Figure 1
In this reaction, one molecule of isobutyraldehyde is treated with the strong base that leads to the formation of a resonance-stabilized enolate ion. Then, this enolate ion reacts with the second molecule of isobutyraldehyde followed by the hydrolysis to form
The product that is formed by the aldol reaction of the given starting material(s) by using
(b)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
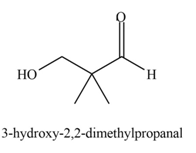
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
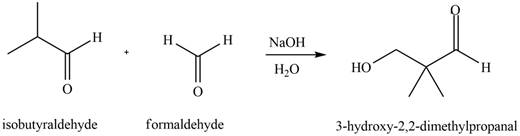
Figure 2
In this reaction, the compound, isobutyraldehyde is treated with the strong base that leads to the formation of a resonance-stabilized enolate ion. Then, this enolate ion reacts with formaldehyde followed by the hydrolysis to form
The product that is formed by the aldol reaction of the given starting material(s) by using
(c)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
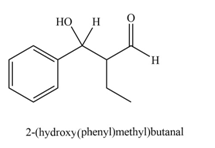
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
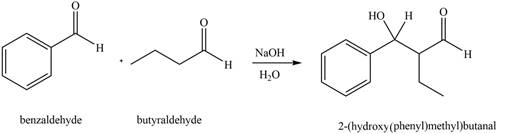
Figure 3
In this reaction, the compound, butyraldehyde is treated with the strong base that leads to the formation of a resonance-stabilized enolate ion. Then, this enolate ion reacts with benzaldehyde followed by the hydrolysis to form
The product that is formed by the aldol reaction of the given starting material(s) by using
(d)
Interpretation: The product that is formed by the aldol reaction of the given starting material(s) by using
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.28P
The product that is formed by the aldol reaction of the given starting material(s) by using
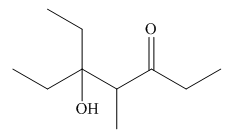
Explanation of Solution
The product that is formed by the aldol reaction of the given starting material(s) by using
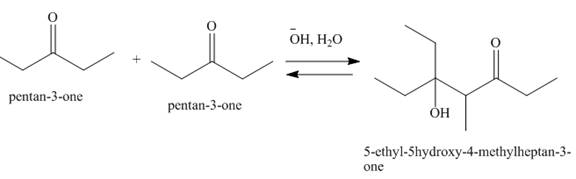
Figure 4
In this reaction, one molecule of pentan-
The product that is formed by the aldol reaction of the given starting material(s) by using
Want to see more full solutions like this?
Chapter 24 Solutions
ALEKS 360 CHEMISTRY ACCESS
- Which statement describes why aldol reactions with ketones are low yielding? A.Ketones do not have acidic protons. B.Ketones are too electron poor at the carbonyl carbon. C.Ketones can attack other ketones well but perform poorly in self-condensations. D.The product of a ketone addition to a ketone has considerable steric strain.arrow_forwardWhat aldehyde or ketone is needed to prepare each compound by an aldol reaction?arrow_forwardDraw the aldol product formed from each compound.arrow_forward
- Aldol Condensation What products are formed? What is the difference between aldol reactions and aldol condensation? What type of reaction condentionas are used?arrow_forwardDraw the final product formed in the reaction below.arrow_forwardThe synthesis of carbohydrates can be particularly difficult because of the large number of chiral centers and OH functional groups present. Epoxides can be useful synthetic intermediates in carbohydrate syntheses. Draw the product of the following reaction of a Gilman reagent with each epoxidearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
