
(a)
Interpretation:
Reasonable precursors have to be suggested for the formation of compound I in the given problem.
Concept introduction:
Amide bond formation:
Amides, commonly known as acid amides are those compounds which have
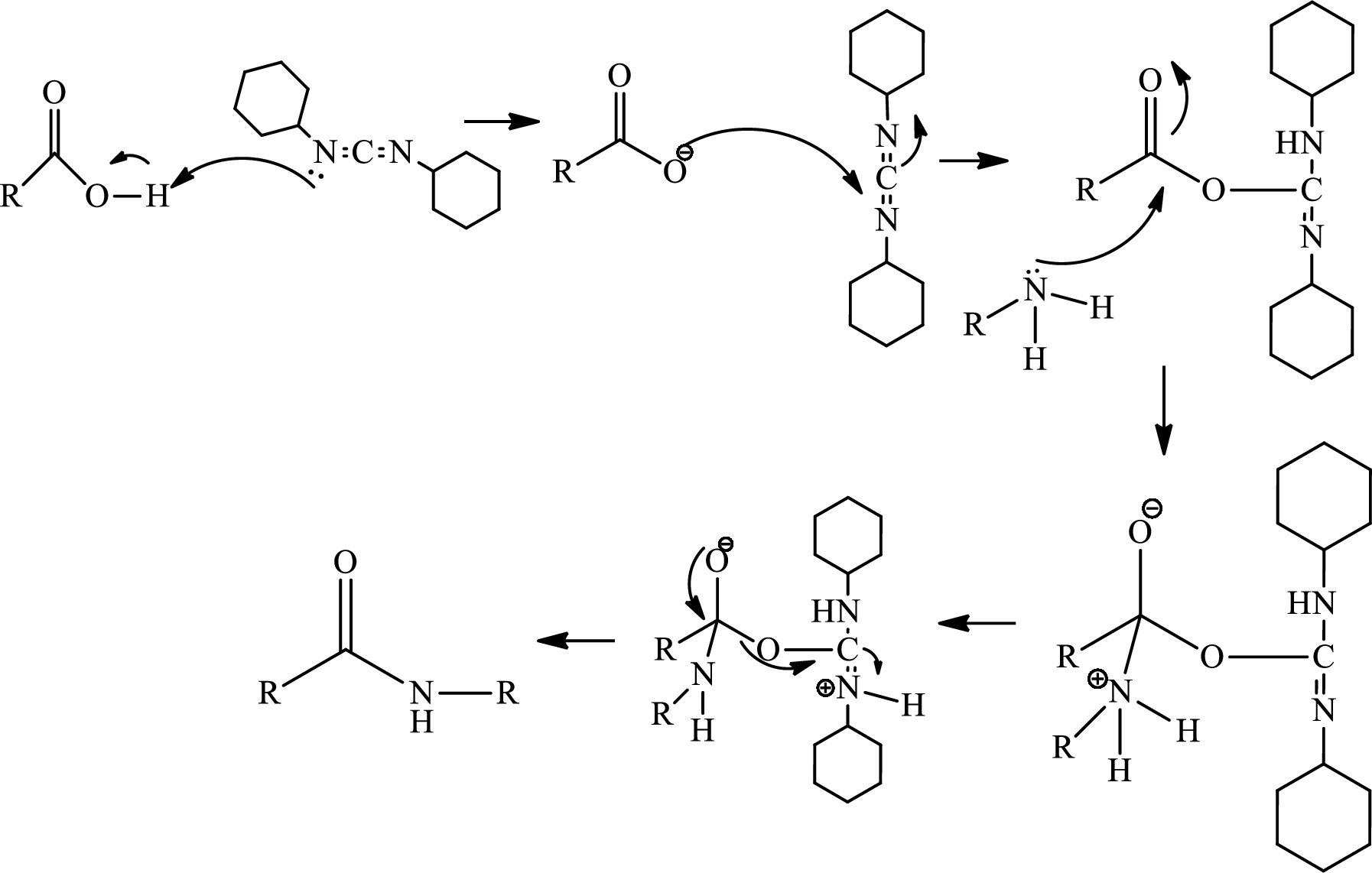
(b)
Interpretation:
Reagents for the reaction along with the mechanism have to be given for the conversion of compound I to II as given in the question.
Concept introduction:
Coupling reaction of aryl halides and phenols catalyzed by palladium and MOP types of ligands:
Palladium catalyzed coupling reactions of aryl halides and phenols are described employing the bulky and electron rich MOP type ligands. When
(c)
Interpretation:
The two isomers of compound II have to be shown.
Concept introduction:
Atropisomers:
Atropisomers are the stereoisomers arising because of the hindered rotation about a single bond where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.
(d)
Interpretation:
Suitable reagents have to be given for the transformation of compound II to III in the given problem.
Concept introduction:
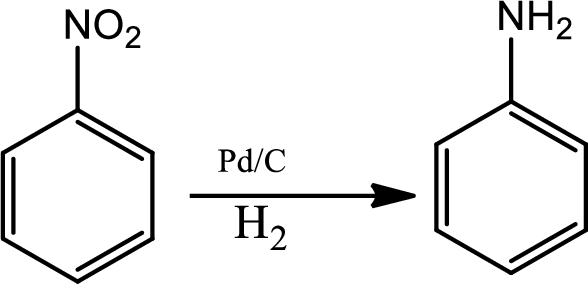
Reduction of nitro group:
Reduction of nitro group can be done by various reagents. In industrial scale reduction of nitrobenzene to anniline is a very common reaction. It is done by catalytic hydrogenation in the presence of palladium hydrogen.
Diazotisation reaction:
Diazonium salta are organic compounds having
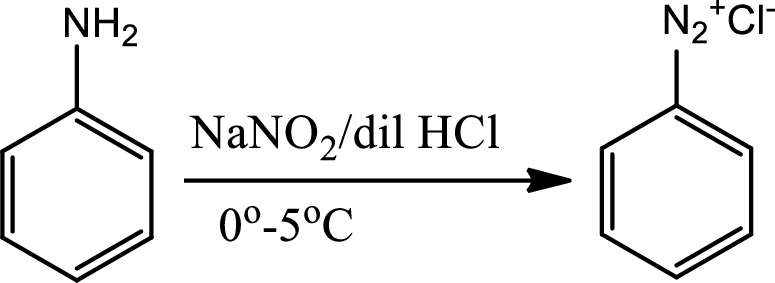
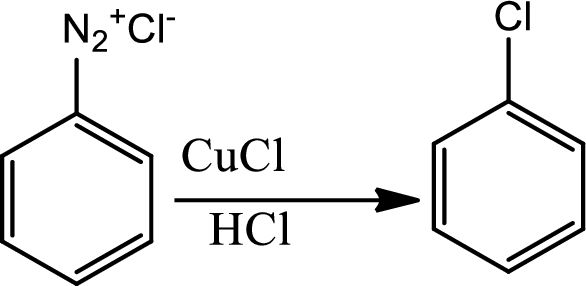
Sandmeyer reaction:
The Sandmeyer reaction is a
(e)
Interpretation:
The reagents and the fragment of the ring A that is useful for the conversion of compound III to IV have to be given.
Concept introduction:
Grignard reaction:
A grignard reagent is a chemical compound with the formula
(f)
Interpretation:
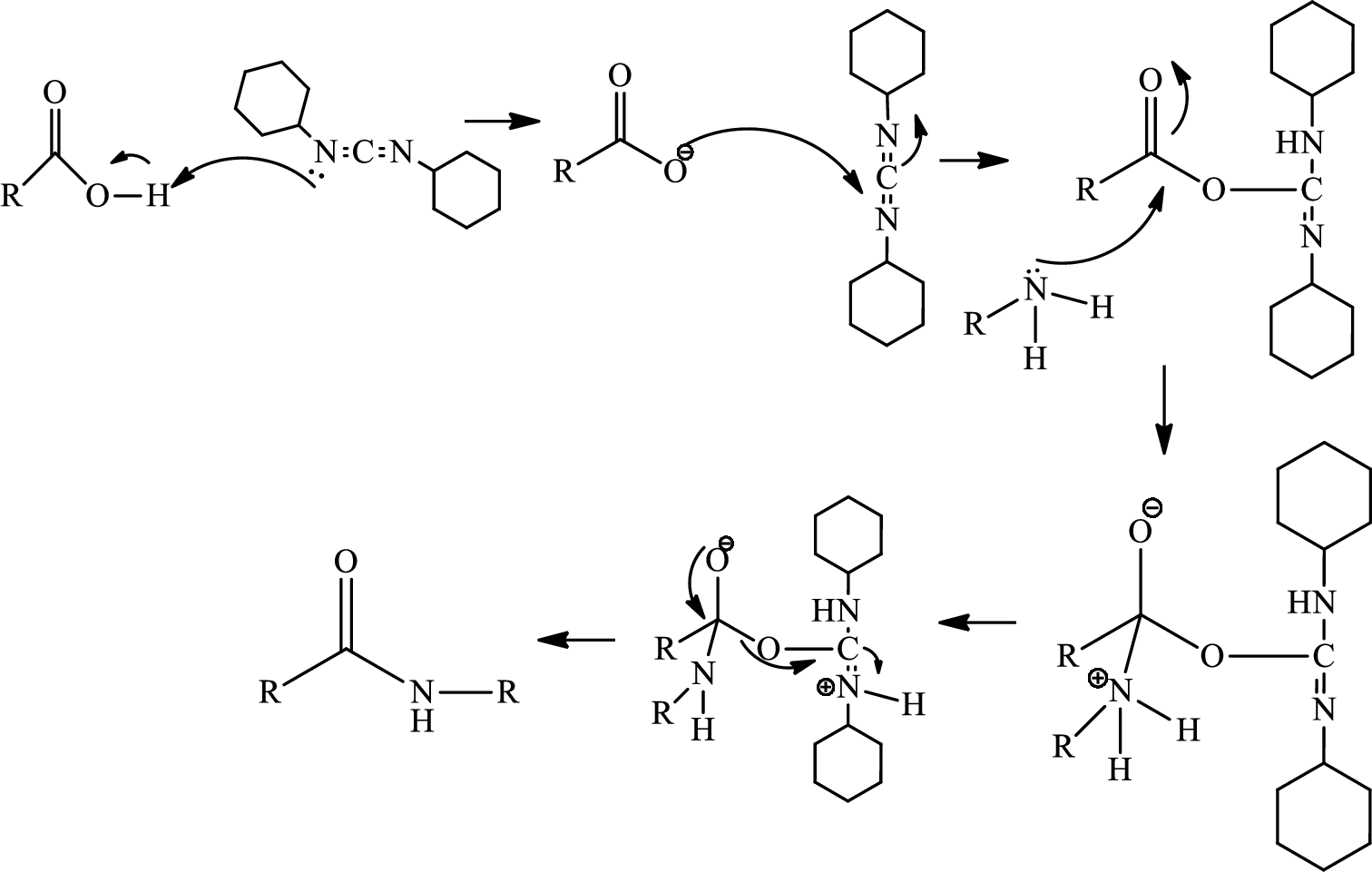
Ring closure reaction of the deprotected free amino group has to be given along with the mechanism.
Concept introduction:
Amide bond formation:
Amides, commonly known as acid amides are those compounds which have
(g)
Interpretation:
The atropisomers have to be drawn and also it has to be shown that only one of them can be converted to vancomycin.
Concept introduction:
Atropisomers:
Atropisomers are the stereoisomers arising because of the hindered rotation about a single bond where energy differences due to steric strain or other contributors create a barrier to rotation that is high enough to allow for isolation of individual conformers.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
ORGANIC CHEMISTRY-OWL V2 ACCESS
- The compound eutypine is an antibacterial agent isolated from the fungus Eutypa lata. This fungus results in a disease common to vineyards called eutyposis. Give a sequence of reactions that will take the following reactant and give eutypine when the other reactants used in the sequence are acetylene and acetone.arrow_forwardDescribe the steps necessary to perform the following synthesisarrow_forwardProstaglandins are a class of eicosanoids, fatty acid derivatives with a variety of extremely potent actions on vertebrate tissues. They are responsible for producing fever and inflammation and its associated pain. Prostaglandins are derived from the 20- carbon fatty acid arachidonic acid in a reaction catalyzed by the enzyme prostaglandin endoperoxide synthase. This enzyme, a cyclooxygenase, uses oxygen to convert arachidonic acid to PGG2, the immediate precursor of many different prostaglandins. Rate of formation of PGG2 with 10 mg/ml ibuprofen (mM/min) Arachidonic acid (mM) Rate of formation of PGG2 (mM/min) 0.190 12.3 0.228 13.9 0.342 17.5 0.570 1.33 22.2 28.8 7.71 8.88 11.9 16.3 24.0 The kinetic data given in the table are for the reaction catalyzed by a mutant of prostaglandin endoperoxide synthase. Focusing here on the first two columns, determine the Vmax and Km of the enzyme. Vmax Km = mM/min mMarrow_forward
- Draw the product formed when phenylacetic acid (C6H5CH2COOH) istreated with following reagent. With some reagents, no reaction occurs. [1] SOCl2; [2] CH3CH2CH2NH2 (excess)arrow_forwardTreatment of ethyl acetoacetate with NaOEt (2 equiv) and BrCH2CH2Br forms compound X. This reaction is the rst step in the synthesis of illudin-S, an antitumor substance isolated from the jack-o’-lantern, a poisonous, saffron-colored mushroom. What is the structure of X?arrow_forwardWhen trichloroacetaldehyde is dissolved in water, almost all of it is converted to the hydrate. Chloral hydrate, the product of the reaction, is a sedative that can be lethal. A cocktail laced with it is known—in detective novels,at least—as a “Mickey Finn.” Explain why an aqueous solution of trichloroacetaldehyde is almost all hydrate.arrow_forward
- Prednisolone is a synthetic corticosteroid that is used as an anti-inflammatory agent. A НО НО HO B Which box contains carbonyl carbon? A Box A B Вох В both ....I ....Iarrow_forwardAspartame, the sweetener used in the commercial products NutraSweet and Equal, is 200 times sweeter than sucrose. What products will be obtained if aspartame is hydrolyzed completely in an aqueous solution of HCl?arrow_forwardAlkylbenzyldimethyl ammonium chloride is a leave-on skin antiseptic used to treat such things as cuts and cold sores. It is also the antiseptic in many hand sanitizers. It is actually a mixture of compounds that differ in the number of carbons (any even number between 8 and 18) in the alkyl group. Show three different sets of reagents (each set composed of an alkyl chloride and an amine) that can be used to synthesize the alkylbenzyldimethyl ammonium chloride shown here.arrow_forward
- (b) Draw a stepwise mechanism for the following transformation from A to B. This reaction is one of the key steps in a synthesis of lysergic acid diethyl amide (aka LSD). CI A CH ₂ AIC13 B CH₂ several steps away from lysergic acid diethyl amide (LSD)arrow_forwardWhat is the reaction mechanism for formaldehyde and phenol under acidic conditions?arrow_forwardDraw the product formed when phenylacetic acid (C6H5CH2COOH) istreated with following reagent. With some reagents, no reaction occurs. [1] SOCl2; [2] (CH3)2CHOHarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
