
Concept explainers
(a)
Interpretation:
Whether hexose corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an
The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The sugar hexose is not the correct description of
Explanation of Solution
Monosaccharide with the six-carbon atom is known as hexose.
The presence of a keto group in
However, the keto group is missing in hexose as shown below.
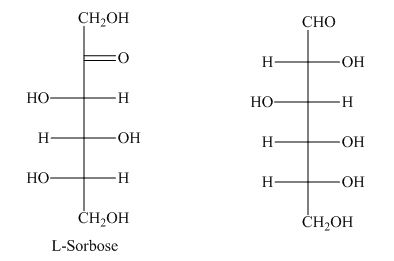
Figure 1
The term hexose is not the correct description of the
(b)
Interpretation:
Whether ketohexose corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The sugar ketohexose is the correct description of
Explanation of Solution
The class of the sugars which is fundamental in carbohydrates with presence of keto group is known as ketohexose sugars.
The presence of keto group in ketohexose shows similarity with
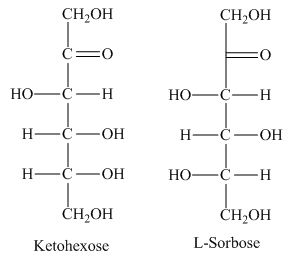
Figure 2
The ketohexose is the correct description of the
(c)
Interpretation:
Whether glycoside molecule corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The carbohydrate
Explanation of Solution
Two molecules of sugar which are connected to a glycosidic bond is known as glycoside molecule. The glycosidic bond is used to join two carbohydrate molecules.
The cyclic acetal group is not present in the
The glycoside molecule is not the correct description of the
(d)
Interpretation:
Whether aldohexose corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The carbohydrate
Explanation of Solution
The aldohexose belongs to the category of hexose in which aldehyde group is present at first carbon. In the
The sugar
(e)
Interpretation:
Whether given structure corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The given structure is the correct description of
Explanation of Solution
The stereocenters are defined as the centers which are chiral in nature or attached with four different substituents. The stereocenters which are in direction of anticlockwise or left-hand nomenclaturehave
The configuration of
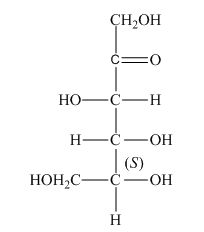
Figure 3
Therefore, the given structure is the correct description of
The given structure shown in Figure 3 is the correct description of
(f)
Interpretation:
Whether given structure corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The given structure is not similar to the
Explanation of Solution
The stereocenters are defined as the centers which are chiral in nature or attached with four different substituents. The stereocenters which are in the direction of anticlockwise or left-hand nomenclature, the configuration of that stereocenter is
The configuration of
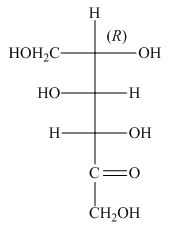
Figure 4
The given structure shown in Figure 4 is not the correct description of
(g)
Interpretation:
Whether given structure corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The given structure is not similar to the
Explanation of Solution
The Haworth projection is used for the arrangements of cyclic sugars. The
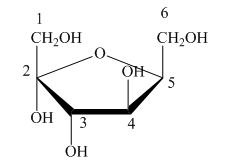
Figure 5
The given structure is not a proper description for
(h)
Interpretation:
Whether given structure corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The given structure is not similar to the
Explanation of Solution
The Haworth projection is used for the arrangements of cyclic sugars. The
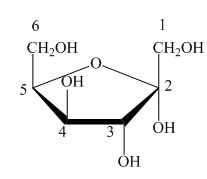
Figure 6
The configuration of sorbose is in
(i)
Interpretation:
Whether given structure corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The given structure is similar to the
Explanation of Solution
The Haworth projection is used for the arrangements of cyclic sugars. The
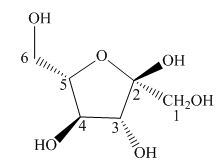
Figure 7
The given structure is the correct description for
(j)
Interpretation:
Whether given structure corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
The pyranoses can be classified as
Answer to Problem 24.40AP
The given structure is not the correct description of
Explanation of Solution
The most stable conformation of cyclohexane is chair form due to the axial and equatorial position. The angle between the carbon-carbon bond is near about
The given structure is not the correct description of
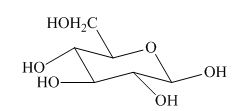
Figure 8
The given structure is not the correct description of
(k)
Interpretation:
Whether given structure corresponds to the correct description of the
Concept introduction:
Carbohydrates are a class of organic compounds. They can be present in the form of open chains or rings. They are usually an aldehyde or ketone with additional hydroxyl groups. The six-membered ring form of carbohydrates is termed as pyranose. The five-membered ring form of carbohydrates is termed as furanose.
Answer to Problem 24.40AP
The given structure is the correct description of
Explanation of Solution
The most stable conformation of cyclohexane is chair form due to the axial and equatorial position. The angle between the carbon-carbon bond is near about
All the substituents or groups are present in the same manner with respect to the
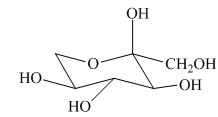
Figure 9
The given structure is the correct description of
Want to see more full solutions like this?
Chapter 24 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- Predict whether d-altrose exists preferentially as a pyranose or a furanose. (Hint: In the most stable arrangement for a five-membered ring, all theadjacent substituents are trans.)arrow_forwarda. What is the classification of the Arabinose in terms of combined no. of carbons and highest functional group present? b. Provide the Cahn-Ingold-Prelog (R.S) Configuration of all the Chiral C present in the structure given above. c. State a Function of arabinose.arrow_forwardd-Altrose is an aldohexose. Ruff degradation of d-altrose gives the same aldopentose asdoes degradation of d-allose, the C3 epimer of glucose. Give the structure of d-altrosearrow_forward
- Is bupropion chiral? If so, how many of the possible stereoisomers are formed in this synthesis?arrow_forwardThe most stable conformation of most aldopyranoses is one in which the largest group, the CH2OH group, is equatorial. However, alpha-D-idopyranose exists primarily in a conformation with an axial CH2OH group. Write formulas for the two chair conformations of a-D-idopyranose (one with the CH2OH group axial and one with the CH2OH group equatorial) and provide an explanationarrow_forwardRuff degradation of d-arabinose gives d-erythrose. The Kiliani–Fischer synthesis converts d-erythrose to a mixture of d-arabinose and d-ribose. Draw out these reactions, andgive the structure of d-ribosearrow_forward
- Thoroughly explain why (a)malthose is a reducing sugar while trehalose is not based on their structures. (b)Why is trehalose very resistant to acid hydrolysis while maltose can be acid-hydrolyzed with ease. Give clear explanations.arrow_forwardDraw the product that is expected when the β-pyranose form of compound A is treated with excess ethyl iodide in the presence of silver oxide. The following information can be used to determine the identity of compound A: The molecular formula of compound A is C6H12O6. Compound A is reducing sugar. When compound A is subjected to a Wohl degradation two times sequentially, Derythrose is obtained. Compound A is epimeric with D-glucose at C3. The configuration at C2 is R.EXPLAIN IN DETAIL.arrow_forwardThat is phenyl butanoate incase it's not cleararrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


