
Concept explainers
a)
Interpretation:
Number of carbon-carbon sigma bonds present in the given benzene has to be identified.
Concept introduction:
Sigma bonds: A sigma bond is a covalent bond formed by head on overlap of atomic orbitals that carries two electrons. The bonding represented as a single line between two atoms.
Head-on overlap:
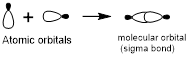
Head-on overlap of two atomic orbitals forms a molecular orbital known as sigma bonds.
Side-to-side overlap:
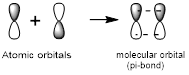
Side-to-side overlap of two atomic orbitals forms a molecular orbital known as pi-bonds.
b)
Interpretation:
Number of carbon-carbon sigma bonds present in the given cyclobutane has to be identified.
Concept introduction:
Sigma bonds: A sigma bond is a covalent bond formed by head on overlap of atomic orbitals that carries two electrons. The bonding represented as a single line between two atoms.
Head-on overlap:
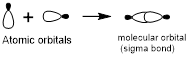
Head-on overlap of two atomic orbitals forms a molecular orbital known as sigma bonds.
Side-to-side overlap:
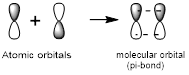
Side-to-side overlap of two atomic orbitals forms a molecular orbital known as pi-bonds.
c)
Interpretation:
Number of carbon-carbon sigma bonds present in the given 3-ethyl-2-methylpentane has to be identified.
Concept introduction:
Sigma bonds: A sigma bond is a covalent bond formed by head on overlap of atomic orbitals that carries two electrons. The bonding represented as a single line between two atoms.
Head-on overlap:
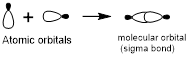
Head-on overlap of two atomic orbitals forms a molecular orbital known as sigma bonds.
Side-to-side overlap:
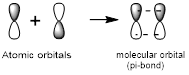
Side-to-side overlap of two atomic orbitals forms a molecular orbital known as pi-bonds.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
CHEMISTRY-ALEK 360 ACCES 1 SEMESTER ONL
- write the structure formulas of alkanes with molecular formula C6H14, which with chlorine give: a) three monochlorinated isomers? b) five monochlorinated isomers c) only two monochlorinated isomersarrow_forwardDefine Ethylene—C2H4 ?arrow_forwardDraw and name the five cycloalkane structures of formula C5H10. Can any of these structures give rise to geometric (cis-trans) isomerism? If so, show the cis and trans stereoisomersarrow_forward
- 1-Propylamine, propan-1-ol, acetic acid, and butane have about the same molar masses. Which would you expect to have the (a) highest boiling point, (b) lowest boiling point, (c) least solubility in water, and (d) least chemical reactivity? Have each member of your group chose a part to answer, andthen discuss with each other why those answers were chosen.arrow_forwardDraw the structures and give the name of the 8 constitutional isomers with the molecular formula C9H12 that contain a benzene ring.arrow_forwardWhat is the skeletal structure pertianing to the IUPAC name for 4-isopropyl-2,4,5, trimethylundecane?arrow_forward
- which functional groups are in C6H12O2 with it having 1 degree of saturation, and is the molecule hexanoic acid?arrow_forwardWhat hydrocarbon with the molecular formula C4H10 forms three monochlorinated products? One is achiral and two are chiral.arrow_forwardName and draw structural formulas for all alkenes with the molecular formula C5H10. As you draw these alkenes, remember that cis and trans isomers are different compounds and must be counted separatelyarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

