
(a)
Interpretation:
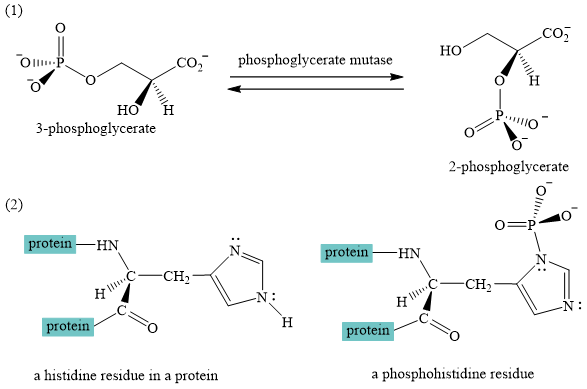
A curved-arrow mechanism for the interconversion of
Concept introduction:
The interconversion of
Answer to Problem 25.35AP
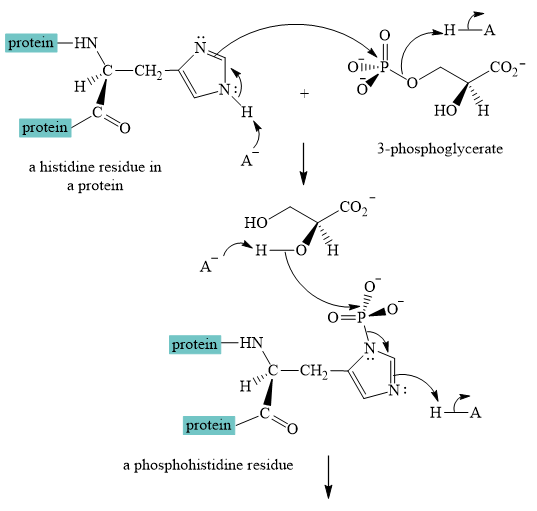
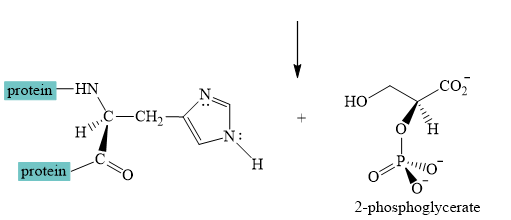
Explanation of Solution
The interconversion equation of
A curved-arrow mechanism for the interconversion of
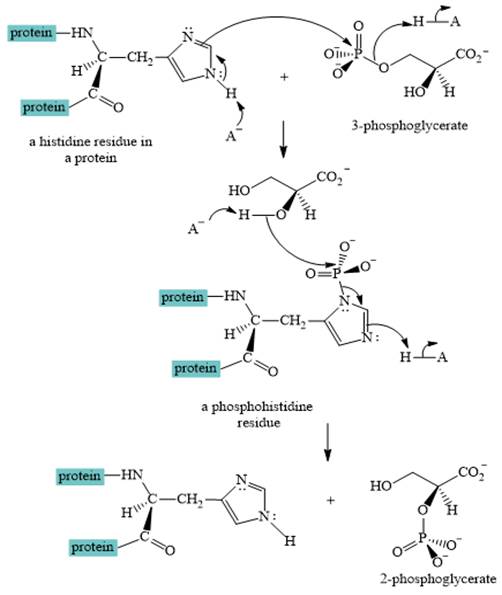
The nitrogen of histidine residue acts as a nucleophile after loss of proton by a base
The two step curved arrow mechanism for the interconversion of
(b)
Interpretation:
If the isotopically chiral
Concept introduction:
The enzyme catalyzed substitution reaction of phosphate ester derivate at phosphate proceeds through
Answer to Problem 25.35AP
If enantiomerically pure
Explanation of Solution
The enzyme catalyzed substitution reaction of phosphate ester derivate at phosphate proceeds through inversion of stereochemistry. The substitution occurs twice with double inversion of the product formed with retention of configuration. Thus, if enantiomerically pure
The interconversion of
Want to see more full solutions like this?
Chapter 25 Solutions
EBK ORGANIC CHEMISTRY STUDY GUIDE AND S
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





