
(a)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction:
1. The hydrocarbon is named after the longest carbon chain.
2. The parent hydrocarbon containing amino group is named as
3. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,
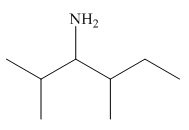
Explanation of Solution
The IUPAC name of the compound is

Figure 1
The structure of the given compound is shown in Figure 1.
(b)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the longest carbon chain.
2. The parent hydrocarbon containing amino group is named as alkane with suffix amine.
3. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,

Explanation of Solution
The IUPAC name of the compound is

Figure 2
The structure of the given compound is shown in Figure 2.
(c)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the longest carbon chain.
2. The parent hydrocarbon containing amino group is named as alkane with suffix amine.
3. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,
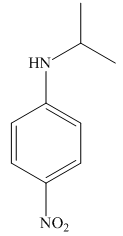
Explanation of Solution
The IUPAC name of the compound is

Figure 3
The structure of the given compound is shown in Figure 3.
(d)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the longest carbon chain.
2. The parent hydrocarbon containing amino group is named as alkane with suffix amine.
3. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,
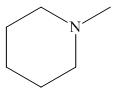
Explanation of Solution
The IUPAC name of the compound is

Figure 4
The structure of the given compound is shown in Figure 4.
(e)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the longest carbon chain.
2. The parent hydrocarbon containing amino group is named as alkane with suffix amine.
3. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,
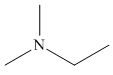
Explanation of Solution
The IUPAC name of the compound is

Figure 5
The structure of the given compound is shown in Figure 5.
(f)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the longest carbon chain.
2. The parent hydrocarbon containing amino group is named as alkane with suffix amine.
3. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,
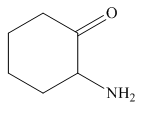
Explanation of Solution
The IUPAC name of the compound is

Figure 6
The structure of the given compound is shown in Figure 6.
(g)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the longest carbon chain.
2. The parent hydrocarbon containing amino group is named as alkane with suffix amine.
3. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,
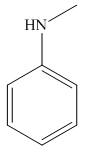
Explanation of Solution
The IUPAC name of the compound is

Figure 7
The structure of the given compound is shown in Figure 7.
(h)
Interpretation: The structure corresponding to given compound is to be drawn.
Concept introduction: IUPAC nomenclature is a systematic way of naming the organic compounds. The basic principles of IUPAC naming for hydrocarbon are:
1. The hydrocarbon is named after the longest carbon chain.
1. The parent hydrocarbon containing amino group is named as alkane with suffix amine.
2. When the nitrogen atom of amine is substituted with alkyl groups, then the amine is named with prefix
Answer to Problem 25.3P
The structure of the given compound is,
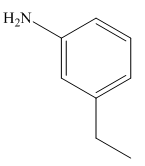
Explanation of Solution
The IUPAC name of the compound is

Figure 8
The structure of the given compound is shown in Figure 8.
Want to see more full solutions like this?
Chapter 25 Solutions
Package: Organic Chemistry with Connect 2-year Access Card
- What are the functional groups present in this antibacterial antibiotic? A. Amide, thioether, aldehyde, phenol, carboxylic acid B. Amide, thioether, ketone, amine, phenol, carboxylic acid C. Amide, thioether, ketone, phenol, carboxylic acid D. Thioether, ketone, amine, phenol, carboxylic acid A brief explanation would be highly appreciated + upvotearrow_forwardMany drugs are sold as their hydrochloride salts (R2NH2 + Cl−), formed by reaction of an amine (R2NH) with HCl . Question: Draw the product (a hydrochloride salt) formed by reaction ofacebutolol with HCl. Acebutolol is a β blocker used to treat high bloodpressure.arrow_forwarda. Explain the effect of acid on the solubility of the water-insoluble amines.arrow_forward
- Reductive amination of carbonyl compounds produces the same kinds of products as does the reaction of amines and alkyl halides True or Falsearrow_forwardDraw the structure that corresponds to each name. a. tri-tert-butylamine b. 2-sec-butylpiperidinearrow_forwardwhy Acetanilide at room temperature is less soluble?arrow_forward
- a. What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer? b. What pH would you make the water layer to cause the carboxylic acid to dissolve in the ether layer and the amine to dissolve in the water layer?arrow_forward1. What are the hazards and benefits of Amines? 2. What are the hazards and benefits of Nitriles? 3. What are the hazards and benefits of Nitro Compounds?arrow_forwardFrom the given structures which is(a) amide that will release a secondary amine upon hydrolysis? (b) product of hydrolysis of MSO (c) a tertiary amide and (d) a diketonearrow_forward
- Which amines cannot be prepared by reduction of an amide?arrow_forwardMatch the description to one of the compounds E– H. a. a compound that contains a 1 ° amine and a 1 ° amide b. a compound that contains a 1 ° amine and a 2 ° amide c. a compound that contains a 2 ° amine and a 3 ° amide d. a compound that contains a 3 ° amine and a 3 ° amidearrow_forwardWhich statements are TRUE?I. Tertiary amines have lower BP than primary and secondary amines.II. Tertiary amines has no possibility for hydrogen bonding.III. Tertiary amines has a high-molecular mass as hydrogen bonding occursarrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




