
Draw the products formed when
a.
b.
c.
d. excess
e.
f.
g.
h.
i. Part (b), then
j.
(a)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
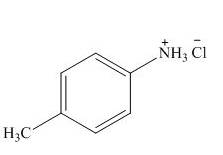
Explanation of Solution
The product formed by the treatment of
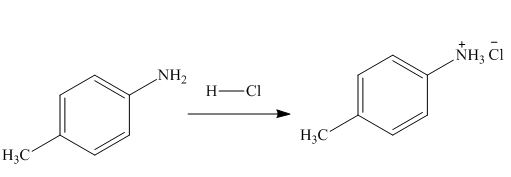
Figure 1
In the above reaction, hydrochloric acid attacks on nitrogen atom of
The product formed by the treatment of
(b)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
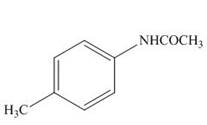
Explanation of Solution
The products formed by the treatment of
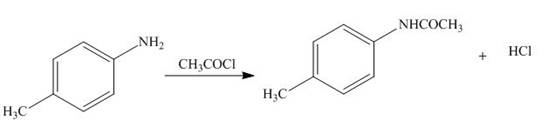
Figure 2
The above reaction indicates that
The products formed by the treatment of
(c)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
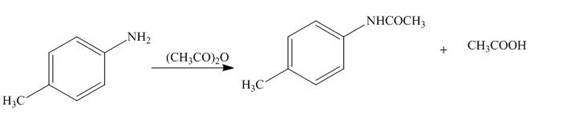
Figure 3
The above reaction implies that
The products formed by the treatment of
(d)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
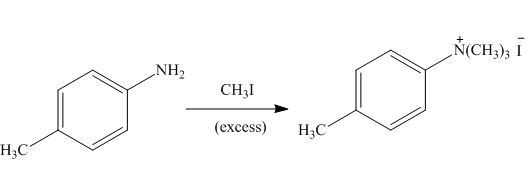
Figure 4
The above reaction indicates that excess of methyl iodide reacts with
The products formed by the treatment of
(e)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
The products formed by the treatment of
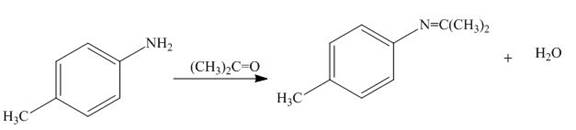
Figure 5
Water gets removed when
The products formed by the treatment of
(f)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The products formed by the treatment of
Explanation of Solution
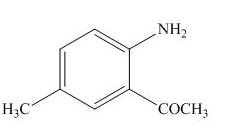
The products formed by the treatment of
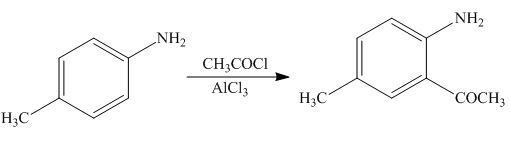
Figure 6
The above reaction indicates that
(g)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
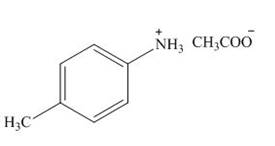
Explanation of Solution
The product formed by the treatment of
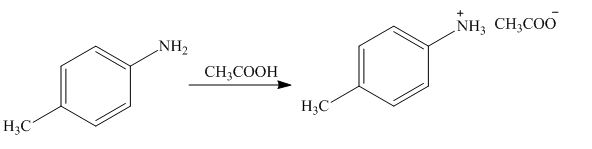
Figure 7
The above reaction indicates that
The product formed by the treatment of
(h)
Interpretation: The products formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
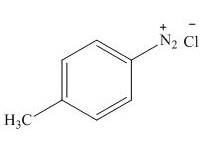
Explanation of Solution
The product formed by the treatment of
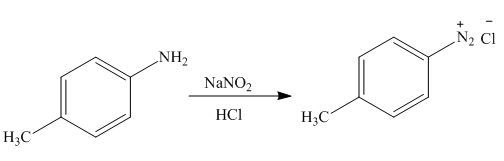
Figure 8
In the above reaction
The product formed by the treatment of
(i)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
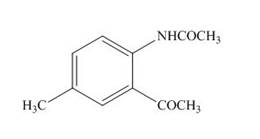
Explanation of Solution
The product formed by the treatment of
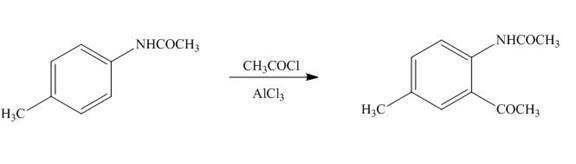
Figure 9
The product formed in part (b) is used as a reactant in the above reaction. It again reacts with
The product formed by the treatment of
(j)
Interpretation: The product formed by the treatment of
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.52P
The product formed by the treatment of
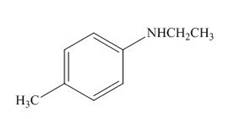
Explanation of Solution
The product formed by the treatment of
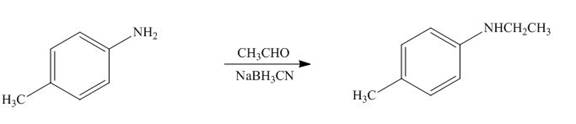
Figure 10
In the above reaction,
The product formed by the treatment of
Want to see more full solutions like this?
Chapter 25 Solutions
ORG.CHEMISTRY CONNECT ACCESS>CUSTOM<
- 2. a. What is the chemical structure of naphthalene, circle functional groups different than alkane,alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? _________________________arrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forwardDraw compounds that contain the following: (a) A primary alcohol (b) A tertiary nitrile (c) A secondary thiol (d) Both primary and secondary alcohols (e) An isopropyl group (f) A quaternary carbonarrow_forward
- Draw the carbonyl products formed when each alcohol is oxidized with K 2Cr 2O 7.arrow_forwarda. What is the chemical structure of 2,6-dichloroindophenol, circle functional groups differentthan alkane, alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? ___________arrow_forwardDraw the product formed when the alcohol cyclobutanol is dehydrated with H2SO4.arrow_forward
- 6. Draw the correct structures for the following: a. what is the correct structure of 3-methyl 1-pentyne? b. what is the correct structure of butyl methyl amine? c. what is the correct structure of 3-methyl 1-pentyne? d. what is the correct structure of 2-methyl 2-butanol? e. what is the correct structure of pentanoic acid?arrow_forwardDraw the product formed when C6H5N2 +Cl− reacts with each compound.arrow_forwardDraw the product formed when C6H5N2+Cl− reacts with each compound.arrow_forward


 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




