
(a)
Interpretation: The products formed when the given amine reacts with [1]
Concept introduction:
Answer to Problem 25.53P
The products formed when the given amine reacts with [1]
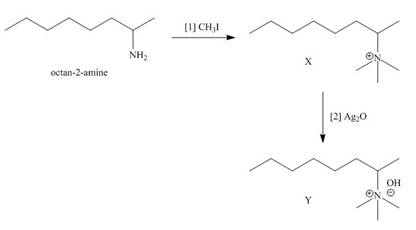
Explanation of Solution
The products formed when the given amine reacts with [1]
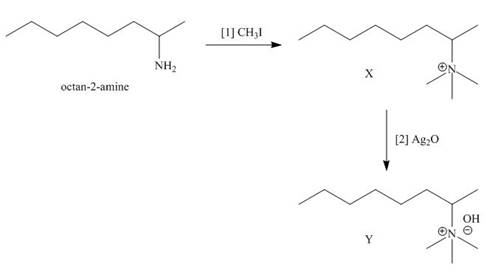
Figure 1
It is conferred from the above reaction that
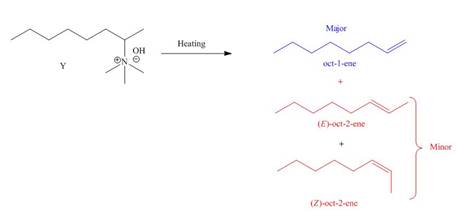
The products formed by heating of compound Y are shown below.
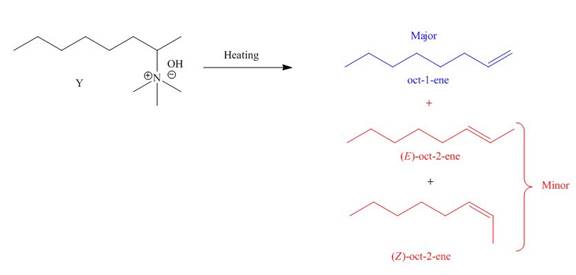
Figure 2
Compound Y on heating undergoes elimination reaction. In the above reaction, base removes
(b)
Interpretation: The products formed when the given amine reacts with [1]
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.53P
The products formed when the given amine reacts with [1]
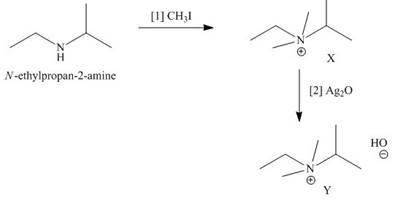
Explanation of Solution
The products formed when the given amine reacts with [1]
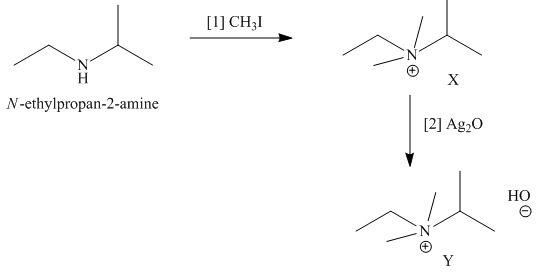
Figure 3
It is conferred from the above reaction that
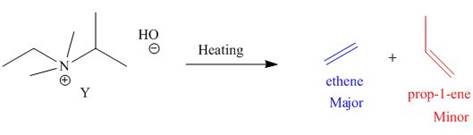
The products formed by heating of compound Y are shown below.
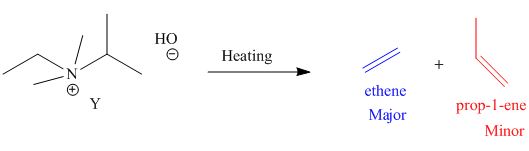
Figure 4
Compound Y on heating undergoes elimination reaction. In the above reaction, base removes
The products formed when the given amine reacts with [1]
(c)
Interpretation: The products formed when the given amine reacts with [1]
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.53P
The products formed when the given amine reacts with [1]
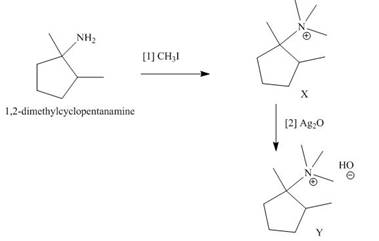
Explanation of Solution
The products formed when the given amine reacts with [1]
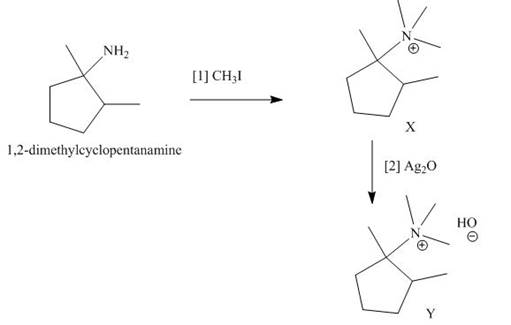
Figure 5
It is conferred from the above reaction that
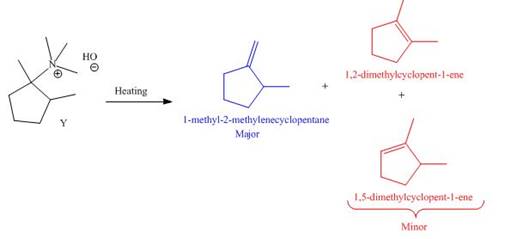
The products formed by heating of compound Y are shown below.
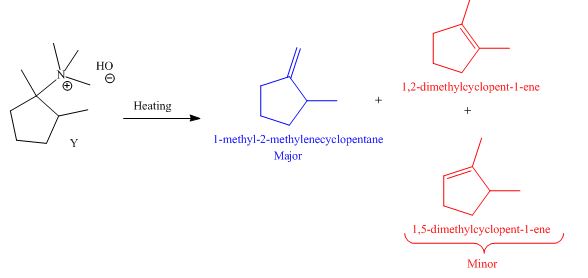
Figure 6
Compound Y on heating undergoes elimination reaction. In the above reaction, base removes
The products formed when the given amine reacts with [1]
(d)
Interpretation: The products formed when the given amine reacts with [1]
Concept introduction: Amines are the derivatives of ammonia consisting of nitrogen atom with the lone pair of electrons. They are basic compounds. The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of molecules with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 25.53P
The products formed when the given amine reacts with [1]
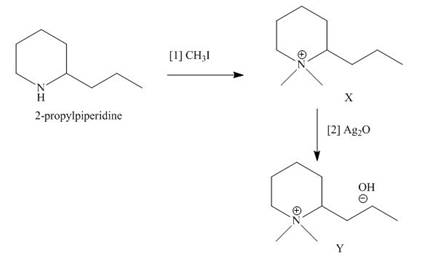
Explanation of Solution
The products formed when the given amine reacts with [1]
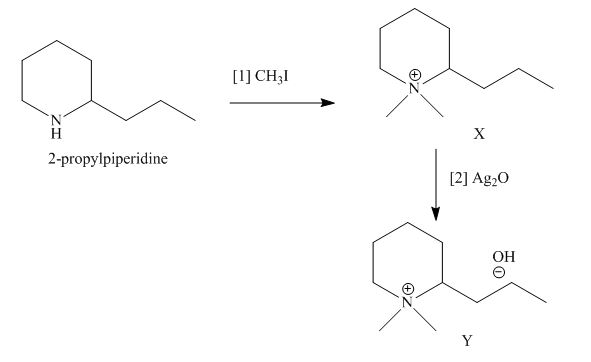
Figure 7
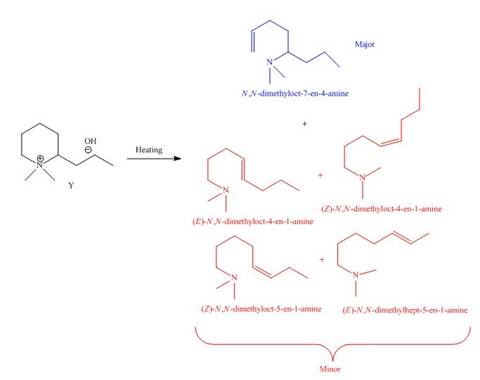
It is conferred from the above reaction that
The products formed by heating of compound Y are shown below.
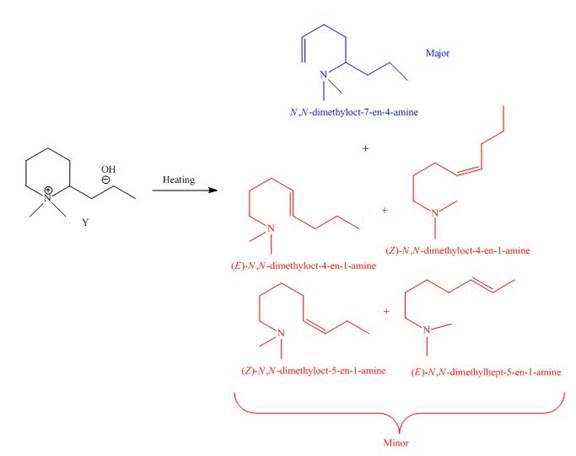
Figure 8
Compound Y on heating undergoes elimination reaction. In the above reaction, base removes
The products formed when the given amine reacts with [1]
Want to see more full solutions like this?
Chapter 25 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
- What carboxylic acid and amine are needed to synthesize the pain reliever phenacetin? Phenacetin was once a component of the over-the-counter pain reliever APC (aspirin, phenacetin, caffeine), but it is no longer used because of its kidney toxicity.arrow_forwardWhat carbonyl compound and amine or alcohol are needed to prepare each product?arrow_forwardStanozolol is an anabolic steroid that promotes muscle growth. Althoughstanozolol has been used by athletes and body builders, many physicaland psychological problems result from prolonged use and it is bannedin competitive sports. Explain why the pKa of the N—H bond in the pyrazole ring iscomparable to the pKa of the O—H bond, making it considerably moreacidic than amines such as CH3NH2 (pKa = 40).arrow_forward
- Write detailed equations showing the mechanism by which aspirin is hydrolyzed in boiling, slightly acidic water.arrow_forwardSafrole, which is isolated from sassafras (Problem 21.33), can be converted to the illegal stimulant MDMA (3,4- methylenedioxymethamphetamine, “Ecstasy”) by a variety of methods. (a) Devise a synthesis that begins with safrole and uses a nucleophilic substitution reaction to introduce the amine. (b) Devise a synthesis that begins with safrole and uses reductive amination to introduce the amine.arrow_forwardThe –NHCOR group of an amide is an activating group, but it is not as strongly activating as NH2. (a) Explain why it is an activating group. (b) Explain why it is less activating than NH2.arrow_forward
- Be sure to answer all parts. Be sure to include the ion charges. What products are formed when the following amine is treated with HCl: (CH3CH2)2NH arrow draw structure ... + Cl− Be sure to answer all parts. What ammonium salt is formed when the amine is treated with HCl.arrow_forwardRank the labeled nitrogen atoms in each compound in order of increasing basicity. Histamine causes the runny nose and watery eyes associated with allergies, and trazodone is a drug used as a sedative and antidepressant.arrow_forwardQ1. How are the alkaloids classified? Q2. Give any four biological sources of quinine? Give the isolation and extraction of quinine. Q3. Give the synthesis of capsaicin from vanillin.arrow_forward
- Explain why the boiling point of propanamide, CH3CH2CONH2, is considerably higher than the boiling point of N,Ndimethylformamide, HCON(CH3)2 (213 °C vs. 153 °C), even though both compounds are isomeric amides.arrow_forwardDraw the products formed when each attached amine is treated with [1] CH3I(excess); [2] Ag2O; [3] Δ. Indicate the major product when a mixtureresults.arrow_forwardDraw the following a) N-methylpropanamideb) cis-2-heptenec) 2-hexanone d) butylbutanoatee) 3-chloropentanoic acid f) 2,2-dibromo-butanalg) 2-octanolh) 1-propynei) butylethylmethylamine j) butylpropyletherarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


