
Concept explainers
(a)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagent that is required to convert acetophenone
Explanation of Solution
The given compound is shown below.
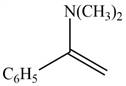
Figure 1
Carbonyl compounds react with secondary
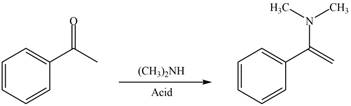
Figure 2
Thus, the reagent that is required to convert acetophenone
The reagent that is required to convert acetophenone
(b)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagent that is required to convert acetophenone
Explanation of Solution
The given compound is shown below.
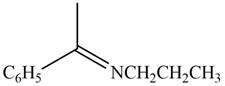
Figure 3
Carbonyl compounds react with primary amines in the presence of mild acid to form imine. Therefore, the reaction that shows the conversion of acetophenone into the given compound is shown below.
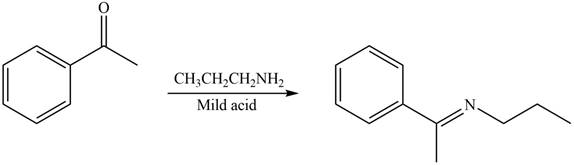
Figure 4
Thus, the reagent that is required to convert acetophenone
The reagent that is required to convert acetophenone
(c)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
he reagents that are required to convert acetophenone
Explanation of Solution
The given compound is shown below.
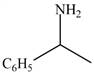
Figure 5
Carbonyl compounds react with ammonia to form imine. The conversion of imine to amines takes place by using selective reducing agent called sodium cyanoborohydride. Therefore, the reaction that shows the conversion of acetophenone into the given compound is shown below.
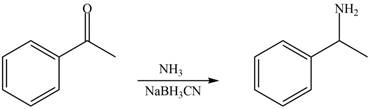
Figure 6
Thus, reagent that is required to convert acetophenone
The reagents that are required to convert acetophenone
(d)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagents that are required to convert acetophenone
Explanation of Solution
The given compound is shown below.
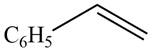
Figure 7
The reaction that shows the conversion of acetophenone into the given compound is shown below.
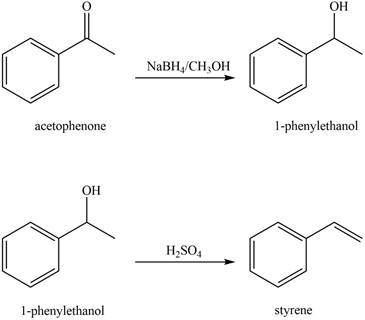
Figure 8
The given reaction is a two step process. The first step involves reduction of carbonyl compounds into alcohol by using sodium borohydride. This results in the formation of
Thus, the reagents that are required to convert acetophenone
The reagents that are required to convert acetophenone
(e)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagents that are required to convert acetophenone
Explanation of Solution
The given compound is shown below.
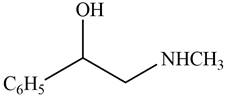
Figure 9
The reaction that shows the conversion of acetophenone into the given compound is shown below.
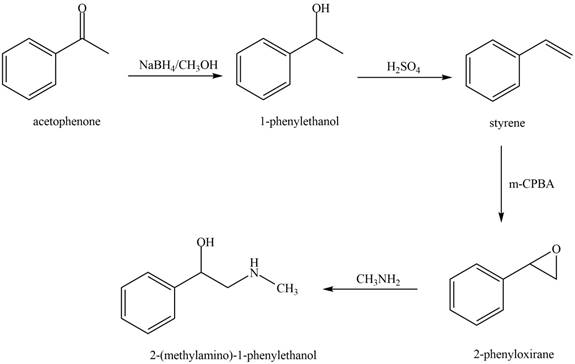
Figure 10
The first step involves reduction of carbonyl compounds into alcohol by using sodium borohydride. This results in the formation of
Thus, the reagents that are required to convert acetophenone
The reagents that are required to convert acetophenone
(f)
Interpretation:
The reagents that are needed to convert acetophenone
Concept introduction:
The structural representation of sugar molecule in cyclic form is known as Haworth projection. A compound in which the hydroxyl group of first and sixth carbon atom is on the same side is known as
Answer to Problem 25.63P
The reagent that is required to convert acetophenone
Explanation of Solution
The given compound is shown below.
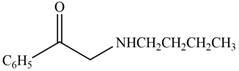
Figure 11
The reaction that shows the conversion of acetophenone into the given compound is shown below.
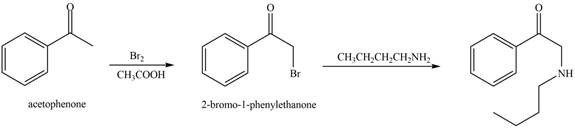
Figure 12
The first step is the bromination step which results in the formation of
Thus, reagent that is required to convert acetophenone
The reagent that is required to convert acetophenone
Want to see more full solutions like this?
Chapter 25 Solutions
ALEKS 360 CHEMISTRY ACCESS
- Although γ-butyrolactone is a biologically inactive compound, it is converted in the body to 4-hydroxybutanoic acid (GHB), an addictive and intoxicating recreational drug. Draw a stepwise mechanism for this conversion in the presence of acid.arrow_forwardDraw a stepwise mechanism for the conversion of lactone C to carboxylic acid D. C is a key intermediate in the synthesis of prostaglandins (Section 19.6) by Nobel Laureate E. J. Corey and co-workers at Harvard University.arrow_forwardWhat lactol (cyclic hemiacetal) is formed from intramolecular cyclization of each hydroxy aldehyde?arrow_forward
- (a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardA key step in the synthesis of the narcotic analgesic meperidine (tradename Demerol) is the conversion of phenylacetonitrile to X. (a) What isthe structure of X? (b) What reactions convert X to meperidine ?arrow_forwarda. acetophenone b. benzaldehyde c. 5% glucose d. acetone e. benzaldehyde f. acetaldehyde which among the 6 samples contains the carbonyl group and which samples doesnt contain the carbonyl group. Explainarrow_forward
- One synthesis of the cholesterol-lowering drug atorvastatin involves the construction of the pyrrole by reaction of diketone X with amine Y. Draw a stepwise mechanism for this reaction.arrow_forwardWhat product is formed when a solution of A and B is treated with mild base? This reaction is the rst step in the synthesis of rosuvastatin (sold as a calcium salt under the trade name Crestor), a drug used to treat patients with high cholesterol.arrow_forwardDraw the organic products formed when allylic alcohol A is treated with each reagent.a.H2 + Pd-C b.mCPBA c. PCC d.CrO3, H2SO4, H2O e.(CH3)3COOH, Ti[OCH(CH3)2]4, (+)-DET f. (CH3)3COOH, Ti[OCH(CH3)2]4, (−)-DET g. [1] PBr3; [2] LiAlH4; [3] H2O h.HCrO4−–Amberlyst A-26 resinarrow_forward
- (a) Explain how NaBH4 in CH3OH can reduce hemiacetal A to butane-1,4-diol (HOCH2CH2CH2CH2OH). (b) What product is formed when A is treated with Ph3P = CHCH2CH(CH3)2? (c) The drug isotretinoin is formed by reaction of X and Y. What is the structure of isotretinoin? Although isotretinoin (trade name Accutane or Roaccutane) is used for the treatment of severe acne, it is dispensed under strict controls because it also causes birth defects.arrow_forwardDraw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form a substituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that can be used as a synthetic detergent to clean away dirt (see Problem 3.22).arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





